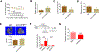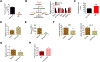Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells - PubMed (original) (raw)
. 2018 Oct 8;34(4):659-673.e6.
doi: 10.1016/j.ccell.2018.08.016. Epub 2018 Sep 27.
Biniam Adane 1, Nabilah Khan 1, Erica Alexeev 2, Nichole Nusbacher 3, Mohammad Minhajuddin 1, Brett M Stevens 1, Amanda C Winters 4, Xi Lin 5, John M Ashton 6, Enkhtsetseg Purev 1, Lianping Xing 5, Daniel A Pollyea 1, Catherine A Lozupone 3, Natalie J Serkova 7, Sean P Colgan 2, Craig T Jordan 8
Affiliations
- PMID: 30270124
- PMCID: PMC6177322
- DOI: 10.1016/j.ccell.2018.08.016
Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells
Haobin Ye et al. Cancer Cell. 2018.
Abstract
From an organismal perspective, cancer cell populations can be considered analogous to parasites that compete with the host for essential systemic resources such as glucose. Here, we employed leukemia models and human leukemia samples to document a form of adaptive homeostasis, where malignant cells alter systemic physiology through impairment of both host insulin sensitivity and insulin secretion to provide tumors with increased glucose. Mechanistically, tumor cells induce high-level production of IGFBP1 from adipose tissue to mediate insulin sensitivity. Further, leukemia-induced gut dysbiosis, serotonin loss, and incretin inactivation combine to suppress insulin secretion. Importantly, attenuated disease progression and prolonged survival are achieved through disruption of the leukemia-induced adaptive homeostasis. Our studies provide a paradigm for systemic management of leukemic disease.
Keywords: IGFBP1; adaptive homeostasis; adipose tissue; insulin resistance; leukemia; microbiota; serotonin; short-chain fatty acids.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Author Contributions: H.Y. and C.T.J. designed the experiments and wrote the paper. H.Y. performed all the experiments. B.A., N.K., E.A. and M.M. helped with animal experiments. N.N., J.M.A. and C.A.L. helped with 16S-rRNA-seq. X.L. and L.X. helped with bone micro-CT. E.P., B.M.S., and D.A.P. helped getting human serum and human BM aspirate samples. N.J.S. directed PET-CT studies and analyzed PET-CT results. B.A., C.A.L. and S.P.C provided critical comments to the manuscript.
Declaration of Interests: The authors declare no competing interests.
Figures
Figure 1.. Leukemia induces IR and reduces serum insulin level.
(A) Insulin tolerance tests (ITT) performed on normal and BN mice (n=8). (B) Glucose utilization by gonadal adipose tissue (GAT) from normal and BN mice at the basal and insulin-stimulated conditions (n=3). (C) Starved normal and BN mice were treated with insulin for 30 min and GAT were harvested for detection of p-Akt. (D) Fasting blood glucose levels in normal and BN mice (n=4). (E) Glucose utilization in normal hematopoietic cells, and leukemia and non-leukemia cells from BN bone marrow. (F) Sorted BN leukemia cells were treated with BSA, insulin (1 ng/ml), IGFBP1 (200 ng/ml), or insulin (1 ng/ml) plus IGFBP1 (200 ng/ml) for 30 min. Cells were harvested for detection of indicated protein. (G) Fasting serum insulin levels in normal, BN and MLL mice (n=5). (H) GAT leukemic burden in type-1 diabetic BN mice (n=5). (I-J) BN mice were treated with insulin. GAT and BM leukemic burden (I), and serum FFAs (J) were examined (n=6). Data are represented as mean ± SD. See also Figure S1.
Figure 2.. Adipose-derived IGFBP1 induces the development of IR in leukemia.
(A) Adipokine arrays on conditioned medium (CM) from normal and BN GAT. Red circle indicates IGFBP1. (B) IGFBP1 levels in CM from normal and BN GAT. (C) IGFBP1 protein levels in GAT, inguinal adipose tissue (IAT) and liver from normal and BN mice. Recombinant (RB) mouse IGFBP1 protein and liver protein extracts were served as positive controls. (D) Serum IGFBP1 levels in normal and BN mice (n=5). (E) Serum IGFBP1 levels in BN mice at different time points after leukemic transplantation (n=4). (F) 3T3-L1 adipocytes were serum starved for 1 hr and then treated with RGD peptides for 30 min. Cells were then treated with insulin (1 ng/ml) and IGFBP1 (200 ng/ml) for 30 min for detection of indicated protein. (G) ITT performed on normal mice treated with IGFBP1 (n=8). (H) Serum IGFBP1 levels in BN mice treated with insulin (n=6).. (I-J) Serum IGF1 levels in normal and BN mice were detected by ELSIA (n=6, I) and immunoblot (J). Data are represented as mean ± SD. See also Figure S2.
Figure 3.. Modulation of IGFBP1 mediates leukemia growth in vivo.
(A) ITT performed on BN mice treated with anti-IGFBP1 antibody (n=8). (B-D) Fasting serum insulin (B), GAT leukemic burden (C) and serum FFAs (D) in BN mice treated with anti-IGFBP1 antibody (n=5). (E) Example images and quantification of femur trabecular bone mass examined by Micro-CT (n=4). (F-G) BM and GAT leukemic burden (F) and fasting serum insulin (G) in BN mice preconditioned with IGFPB1 (n=5). Data are represented as mean ±SD. See also Figure S3.
Figure 4.. Increased DPP4 and loss of active GLP-1 contribute to the inhibition of insulin secretion in leukemia pathogenesis.
(A) Immunofluorescent staining for insulin in pancreas from normal, BN and MLL mice. (B) Glucose stimulated insulin secretion (GSIS) performed on normal, BN and MLL mice (n=4). (C) Glucose tolerance test (GTT) performed on normal and BN mice (n=6). (D-E) Fasting serum DPP4 (D) and active GLP-1 levels (E) in normal and BN mice (n=5). (F-I) Fasting serum insulin (F), serum IGFBP1 (G), serum FFAs (H), and BM and GAT leukemic burden (I) in exenatide treated BN mice (n=6). Data are represented as mean ± SD. See also Figure S4.
Figure 5.. Loss of serotonin leads to the inhibition of insulin secretion in leukemia pathogenesis.
(A) Serotonin levels in normal and BN mice (n=4). (B) Expression of gene involved in serotonin metabolism in the colon tissues from normal and BN mice. (C) GI transition time in normal and BN mice (n=5). (D-G) Fasting serum insulin (D), serum IGFBP (E), GAT and BM leukemic burden (F), and serum FFAs (G) in serotonin treated BN mice (n=5). (H) Serum serotonin levels in anti-IGFBP1 antibody treated BN mice (n=5). Data are represented as mean ± SD. See also Figure S5.
Figure 6.. Leukemia-associated microbiota facilitates disease progression.
(A) Expression of anti-microbial genes in the colon tissues from normal and BN mice. (B) 16S-rRNA-sequencing was performed on the fecal materials collected from normal and BN mice (day 13 after leukemic transplantation). Circle indicates Bacteroidales S24–7. (C-E) BM and GAT leukemia burden (C), serum IGFBP1 (D), and fasting serum insulin (E) in BN mice transplanted with fecal materials from normal or BN mice (n=7). (F) BM and GAT leukemic burden in BN mice treated with Abx (n=5). (G) Serum IGFBP1 levels in BN mice treated with Abx (n=5). (H) ITT performed on nonleukemic mice transplanted with normal or BN fecal materials (n=8). (I) Fasting blood glucose and fasting serum insulin levels in non-leukemic mice transplanted with normal or BN fecal materials (n=7). Data are represented as mean ± SD. See also Figure S6 and Table S1.
Figure 7.. Microbiota-derived short chain fatty acids (SCFAs) impede leukemia progression and are reduced in leukemic mice.
(A) Serum FITC-Dextran levels in normal and BN mice (n=5). (B) Hematoxylin and Eosin (H&E, left two images) staining and GFP (for leukemia cells, right two images) staining of colon tissues from normal and BN mice. (C) The amount of butyrate and propionate in fecal samples from normal and BN mice (n=8). (D-G) Serum FITCDextran (D), BM and GAT leukemic burden (E), serum IGFBP1 (F), and fasting serum insulin (G) in tributyrin treated BN mice (n=6). (H) BM and GAT leukemic burden in BN mice treated with combination of tributyrin and serotonin (Ser-Tri) (n=7). (I) Survival curve for BN mice treated with Ser-Tri therapy (tributyrin and serotonin) (n=8). (J) A cartoon showing the experimental procedure and the glucose utilization in vehicle treated and Ser-Tri therapy treated (one-time treatment) leukemic mice (n=4). (K) Representative images of 18F-fluorodeoxyglucose (FDG) uptake by PET-CT scanning in normal mice, untreated and Ser-Tri treated BN mice. Normalized uptake values fold change examined by FDG-PET are presented in the graph (n=4). (L)Schematic representation of the treatment protocol and the survival curve for BN mice treated with chemotherapy alone or chemotherapy combined with the Ser-Tri therapy (n=9). Data are represented as mean ± SD. See also Figure S7.
Figure 8.. Human leukemia induces an insulin resistant phenotype.
(A) Serum IGFBP1 levels in normal controls (n=6), MDS (n=10), and AML (n=13) patients. (B) IGFBP1 level in normal (n=5) and AML (n=5) BM aspirate. (C) IGFBP1 levels in paired BM aspirates from newly diagnostic, remission, and relapsed AML patients. (D) Cytokine arrays performed on serum samples from normal controls and AML patients. (E) Serum FFA levels in normal controls, and MDS and AML patients. (F) ITT performed on NSG mice transplanted with a human primary leukemia sample (n=8). (G) Serum IGFBP1 levels in normal NSG mice and NSG mice transplanted with a human primary leukemia sample (n=8). (H) Serum serotonin levels in normal controls, and MDS and AML patients. (I) Serotonin levels in paired BM aspirates from newly diagnostic, remission and relapsed AML patients. (J-K) Serum insulin levels (J) and serum glycated protein levels (K) in normal controls, and MDS and AML patients. (L) Schematic summary of system-wide perturbations leading to altered glucose utilization in leukemia. Data are represented as mean ± SD. See also Figure S8.
Comment in
- Sabotaging the host.
Baratta MG. Baratta MG. Nat Rev Cancer. 2018 Dec;18(12):722-723. doi: 10.1038/s41568-018-0078-4. Nat Rev Cancer. 2018. PMID: 30385874 No abstract available. - Parasitic Behavior of Leukemic Cells in Systemic Host Metabolism.
Leca J, Berger T, Mak TW. Leca J, et al. Cell Metab. 2018 Dec 4;28(6):811-813. doi: 10.1016/j.cmet.2018.11.011. Cell Metab. 2018. PMID: 30517895
Similar articles
- Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota.
Janssen AWF, Katiraei S, Bartosinska B, Eberhard D, Willems van Dijk K, Kersten S. Janssen AWF, et al. Diabetologia. 2018 Jun;61(6):1447-1458. doi: 10.1007/s00125-018-4583-5. Epub 2018 Mar 3. Diabetologia. 2018. PMID: 29502266 Free PMC article. - Loss of ovarian function in association with a high-fat diet promotes insulin resistance and disturbs adipose tissue immune homeostasis.
Pae M, Baek Y, Lee S, Wu D. Pae M, et al. J Nutr Biochem. 2018 Jul;57:93-102. doi: 10.1016/j.jnutbio.2018.03.011. Epub 2018 Mar 20. J Nutr Biochem. 2018. PMID: 29680663 - Loss of BMP receptor type 1A in murine adipose tissue attenuates age-related onset of insulin resistance.
Schulz TJ, Graja A, Huang TL, Xue R, An D, Poehle-Kronawitter S, Lynes MD, Tolkachov A, O'Sullivan LE, Hirshman MF, Schupp M, Goodyear LJ, Mishina Y, Tseng YH. Schulz TJ, et al. Diabetologia. 2016 Aug;59(8):1769-77. doi: 10.1007/s00125-016-3990-8. Epub 2016 May 21. Diabetologia. 2016. PMID: 27209464 Free PMC article. - Role of glucocorticoids in the physiopathology of excessive fat deposition and insulin resistance.
Asensio C, Muzzin P, Rohner-Jeanrenaud F. Asensio C, et al. Int J Obes Relat Metab Disord. 2004 Dec;28 Suppl 4:S45-52. doi: 10.1038/sj.ijo.0802856. Int J Obes Relat Metab Disord. 2004. PMID: 15592486 Review. - Hypothalamic Insulin Resistance in Obesity: Effects on Glucose Homeostasis.
Chen W, Balland E, Cowley MA. Chen W, et al. Neuroendocrinology. 2017;104(4):364-381. doi: 10.1159/000455865. Epub 2017 Jan 26. Neuroendocrinology. 2017. PMID: 28122381 Review.
Cited by
- Metabolic dependencies and vulnerabilities in leukemia.
Rashkovan M, Ferrando A. Rashkovan M, et al. Genes Dev. 2019 Nov 1;33(21-22):1460-1474. doi: 10.1101/gad.326470.119. Genes Dev. 2019. PMID: 31676734 Free PMC article. Review. - Systematic Construction and Validation of a Metabolic Risk Model for Prognostic Prediction in Acute Myelogenous Leukemia.
Wang Y, Hu F, Li JY, Nie RC, Chen SL, Cai YY, Shu LL, Deng DJ, Xu JB, Liang Y. Wang Y, et al. Front Oncol. 2020 Apr 21;10:540. doi: 10.3389/fonc.2020.00540. eCollection 2020. Front Oncol. 2020. PMID: 32373530 Free PMC article. - Proton export alkalinizes intracellular pH and reprograms carbon metabolism to drive normal and malignant cell growth.
Man CH, Mercier FE, Liu N, Dong W, Stephanopoulos G, Jiang L, Jung Y, Lin CP, Leung AYH, Scadden DT. Man CH, et al. Blood. 2022 Jan 27;139(4):502-522. doi: 10.1182/blood.2021011563. Blood. 2022. PMID: 34610101 Free PMC article. - Comorbidity of Anxiety and Hypertension: Common Risk Factors and Potential Mechanisms.
Qiu T, Jiang Z, Chen X, Dai Y, Zhao H. Qiu T, et al. Int J Hypertens. 2023 May 25;2023:9619388. doi: 10.1155/2023/9619388. eCollection 2023. Int J Hypertens. 2023. PMID: 37273529 Free PMC article. Review. - Advances in Understanding the Links between Metabolism and Autophagy in Acute Myeloid Leukemia: From Biology to Therapeutic Targeting.
Saulle E, Spinello I, Quaranta MT, Labbaye C. Saulle E, et al. Cells. 2023 Jun 5;12(11):1553. doi: 10.3390/cells12111553. Cells. 2023. PMID: 37296673 Free PMC article. Review.
References
- Barnes KM, and Miner JL (2009). Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Pept Sci 10, 96–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA166265/CA/NCI NIH HHS/United States
- R01 CA220986/CA/NCI NIH HHS/United States
- F31 CA196330/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- S10 OD023485/OD/NIH HHS/United States
- R37 DK050189/DK/NIDDK NIH HHS/United States
- R01 DK104713/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous