PET imaging of [11C]PBR28 in Parkinson's disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding - PubMed (original) (raw)
PET imaging of [11C]PBR28 in Parkinson's disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding
Katarina Varnäs et al. Eur J Nucl Med Mol Imaging. 2019 Feb.
Abstract
Purpose: To examine the hypothesis that cerebral binding to the 18 kDa translocator protein (TSPO), a marker of microglia activation, is elevated in Parkinson's disease (PD), and to assess the relationship between brain TSPO binding and dopaminergic pathology in PD.
Methods: The radioligand [11C]PBR28 was used for quantitative assessment of brain TSPO in 16 control subjects and 16 PD patients. To analyse the relationship between dopaminergic pathology and brain TSPO binding, PET studies of the dopamine transporter (DAT) were undertaken in PD patients using the DAT radioligand [18F]FE-PE2I. The total distribution volume of [11C]PBR28 was quantified in nigrostriatal regions, limbic cortices and thalamus, and the binding potential of [18F]FE-PE2I was quantified in nigrostriatal regions.
Results: Based on genotype analysis of the TSPO rs6971 polymorphism, 16 subjects (8 control subjects and 8 PD patients) were identified as high-affinity binders, and the remaining subjects were identified as mixed-affinity binders. A two-way ANOVA showed a strong main effect of TSPO genotype on the cerebral binding of [11C]PBR28, whereas no statistically significant main effect of diagnostic group, or a group by genotype interaction was found for any of the regions analysed. [18F]FE-PE2I PET studies in patients indicated a marked reduction in nigrostriatal binding to DAT. However, no correlations between the binding parameters were found for [11C]PBR28 and [18F]FE-PE2I.
Conclusion: The findings do not support the hypothesis of elevated cerebral TSPO binding or a relationship between TSPO binding and dopaminergic pathology in PD.
Keywords: 18 kDa translocator protein; Dopamine transporter; PET imaging; Parkinson’s disease.
Conflict of interest statement
Conflicts of interest
The studies were supported by AstraZeneca. Dr. Cselényi, Dr. Jucaite and Prof. Farde are employees of AstraZeneca. Prof. Farde has served as a panel member for evaluation of the research programs of the Faculty of Medicine, University of Helsinki, Finland. The other authors declare no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
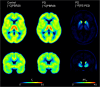
Fig. 1
Average parametric images of [11C]PBR28 _V_T in control subjects (left) and PD patients (centre) and of [18F]FE-PE2I BPND in PD patients (right). The areas with high [11C]PBR28 _V_T represent binding in the thalamus
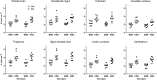
Fig. 2
[11C]PBR28 _V_T values of selected brain regions in control subjects and PD patients. MAB mixed-affinity binder, HAB high-affinity binder
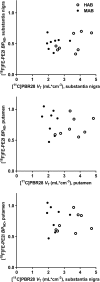
Fig. 3
Correlations between [11C]PBR28 _V_T and [18F]FE-PE2I BPND in PD patients for the substantia nigra (top) and putamen (centre), and between [18F]FE-PE2I BPND for the putamen and [11C]PBR28 _V_T for the substantia nigra (bottom). MAB mixed-affinity binder, HAB high-affinity binder
Similar articles
- Quantitative Analysis of ¹⁸F-(E)-N-(3-Iodoprop-2-Enyl)-2β-Carbofluoroethoxy-3β-(4'-Methyl-Phenyl) Nortropane Binding to the Dopamine Transporter in Parkinson Disease.
Fazio P, Svenningsson P, Forsberg A, Jönsson EG, Amini N, Nakao R, Nag S, Halldin C, Farde L, Varrone A. Fazio P, et al. J Nucl Med. 2015 May;56(5):714-20. doi: 10.2967/jnumed.114.152421. Epub 2015 Mar 19. J Nucl Med. 2015. PMID: 25791993 - Influence of alcoholism and cholesterol on TSPO binding in brain: PET [11C]PBR28 studies in humans and rodents.
Kim SW, Wiers CE, Tyler R, Shokri-Kojori E, Jang YJ, Zehra A, Freeman C, Ramirez V, Lindgren E, Miller G, Cabrera EA, Stodden T, Guo M, Demiral ŞB, Diazgranados N, Park L, Liow JS, Pike V, Morse C, Vendruscolo LF, Innis RB, Koob GF, Tomasi D, Wang GJ, Volkow ND. Kim SW, et al. Neuropsychopharmacology. 2018 Aug;43(9):1832-1839. doi: 10.1038/s41386-018-0085-x. Epub 2018 May 3. Neuropsychopharmacology. 2018. PMID: 29777199 Free PMC article. - Optimal Acquisition Time Window and Simplified Quantification of Dopamine Transporter Availability Using 18F-FE-PE2I in Healthy Controls and Parkinson Disease Patients.
Sonni I, Fazio P, Schain M, Halldin C, Svenningsson P, Farde L, Varrone A. Sonni I, et al. J Nucl Med. 2016 Oct;57(10):1529-1534. doi: 10.2967/jnumed.115.171231. Epub 2016 May 26. J Nucl Med. 2016. PMID: 27230923 - Neuroinflammation in Parkinson's disease: a meta-analysis of PET imaging studies.
Zhang PF, Gao F. Zhang PF, et al. J Neurol. 2022 May;269(5):2304-2314. doi: 10.1007/s00415-021-10877-z. Epub 2021 Nov 1. J Neurol. 2022. PMID: 34724571 Review.
Cited by
- Early stopping in clinical PET studies: How to reduce expense and exposure.
Svensson JE, Schain M, Knudsen GM, Ogden RT, Plavén-Sigray P. Svensson JE, et al. J Cereb Blood Flow Metab. 2021 Nov;41(11):2805-2819. doi: 10.1177/0271678X211017796. Epub 2021 May 21. J Cereb Blood Flow Metab. 2021. PMID: 34018825 Free PMC article. Review. - Have (R)-[11C]PK11195 challengers fulfilled the promise? A scoping review of clinical TSPO PET studies.
Chauveau F, Becker G, Boutin H. Chauveau F, et al. Eur J Nucl Med Mol Imaging. 2021 Dec;49(1):201-220. doi: 10.1007/s00259-021-05425-w. Epub 2021 Aug 13. Eur J Nucl Med Mol Imaging. 2021. PMID: 34387719 Free PMC article. Review. - PET imaging of neuroinflammation in neurological disorders.
Kreisl WC, Kim MJ, Coughlin JM, Henter ID, Owen DR, Innis RB. Kreisl WC, et al. Lancet Neurol. 2020 Nov;19(11):940-950. doi: 10.1016/S1474-4422(20)30346-X. Lancet Neurol. 2020. PMID: 33098803 Free PMC article. Review. - Glia Imaging Shows Clinical Utility in Differentiating Parkinson's Disease from Multiple System Atrophy.
Rinne JO, Jucaite A, Cselényi Z, Farde L. Rinne JO, et al. Mov Disord. 2022 Aug;37(8):1776-1778. doi: 10.1002/mds.29078. Epub 2022 Jun 6. Mov Disord. 2022. PMID: 35666059 Free PMC article. No abstract available. - NRM 2021 Abstract Booklet.
[No authors listed] [No authors listed] J Cereb Blood Flow Metab. 2021 Dec;41(1_suppl):11-309. doi: 10.1177/0271678X211061050. J Cereb Blood Flow Metab. 2021. PMID: 34905986 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical