Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues - PubMed (original) (raw)
Review
Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues
Ziyi Song et al. Nutrients. 2018.
Abstract
De novo lipogenesis (DNL) is a complex and highly regulated process in which carbohydrates from circulation are converted into fatty acids that are then used for synthesizing either triglycerides or other lipid molecules. Dysregulation of DNL contributes to human diseases such as obesity, type 2 diabetes, and cardiovascular diseases. Thus, the lipogenic pathway may provide a new therapeutic opportunity for combating various pathological conditions that are associated with dysregulated lipid metabolism. Hepatic DNL has been well documented, but lipogenesis in adipocytes and its contribution to energy homeostasis and insulin sensitivity are less studied. Recent reports have gained significant insights into the signaling pathways that regulate lipogenic transcription factors and the role of DNL in adipose tissues. In this review, we will update the current knowledge of DNL in white and brown adipose tissues with the focus on transcriptional, post-translational, and central regulation of DNL. We will also summarize the recent findings of adipocyte DNL as a source of some signaling molecules that critically regulate energy metabolism.
Keywords: ChREBP; FASN; LXR; SREBP; adipocyte; central regulation; de novo lipogenesis; insulin resistance; obesity; post-translation; thermogenesis; transcription.
Conflict of interest statement
The authors declare no conflicts of interest with the contents of this article.
Figures
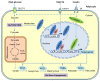
Figure 1
Transcriptional activation of de novo lipogenesis in adipocytes in response to high-sugar or high-fat diets. After the consumption of carbohydrates, a portion of the circulating glucose is taken by adipocytes through insulin-stimulated GLUT4, and then through glycolysis in the cytosol, glucose is converted to pyruvate, which is transported into mitochondria for further oxidation in the tricarboxylic acid (TCA) cycle. Citrate, an intermediate of the TCA cycle, is exported into cytosol and used as a substrate for de novo lipogenesis. Regulation of lipogenesis is mainly at the transcriptional level and carbohydrate response element-binding protein (ChREBP) plays a major role in adipocyte lipogenesis. Glucose metabolites generated during glycolysis activate ChREBP-α, which, together with Max-like protein X (MLX), binds to the carbohydrate response elements (ChoRE) in the promoters of target genes, including those encoding ATP-citrate lyase (ACLY), acetyl-CoA carboxylases 1 (ACC1), fatty acid synthase (FASN), stearoyl-CoA desaturase-1 (SCD1), and ChREBP-β. The induced ChREBP-β in turn further activates its target gene expression, which ultimately promotes the synthesis of fatty acids. Another lipogenic transcription factor sterol regulatory element-binding protein-1 (SREBP-1), induced by insulin at multiple levels, may play a minor role in adipocyte lipogenesis. Compared with carbohydrates, fat consumption inhibits de novo lipogenesis in adipocyte mainly through blocking the activation of ChREBP-β. FATP—fatty acid transport protein-1; IR—insulin receptor.
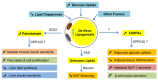
Figure 2
Systemic effects of lipokines produced by de novo lipogenesis in adipocytes. When de novo lipogenesis is increased in adipocytes by means of increasing glucose uptake, decreasing lipid chaperones, or others, some bioactive fatty acids such as palmitoleate and fatty acid ester of hydroxyl fatty acids (FAHFAs) are produced. As a product of SCD1 in adipocytes, palmitoleate functions to improve insulin sensitivity in skeletal muscle and liver, promote pancreatic β-cell proliferation, and inhibit lipid synthesis in the liver. Although it is unclear how FAHFAs are synthesized in adipocytes, these lipids have a function to stimulate adipocyte glucose uptake, intestinal glucagon-like peptide-1 (GLP-1) secretion and β-cell insulin secretion, and reduce inflammation in adipose tissues. The metabolically beneficial effects of palmitoleate and FAHFAs are probably through G protein-coupled receptor 120 (GPR120). In addition, FASN may produce some unknown lipid products in white adipocytes that function to inhibit white fat browning through neuronal circuit regulation. WAT—white adipose tissues.
Similar articles
- Wnt/β-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells.
Bagchi DP, Nishii A, Li Z, DelProposto JB, Corsa CA, Mori H, Hardij J, Learman BS, Lumeng CN, MacDougald OA. Bagchi DP, et al. Mol Metab. 2020 Dec;42:101078. doi: 10.1016/j.molmet.2020.101078. Epub 2020 Sep 9. Mol Metab. 2020. PMID: 32919095 Free PMC article. - Brown Fat AKT2 Is a Cold-Induced Kinase that Stimulates ChREBP-Mediated De Novo Lipogenesis to Optimize Fuel Storage and Thermogenesis.
Sanchez-Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Martinez Calejman C, Gujja S, Li H, Edwards YJK, Wolfrum C, Metallo CM, Nielsen S, Scheele C, Guertin DA. Sanchez-Gurmaches J, et al. Cell Metab. 2018 Jan 9;27(1):195-209.e6. doi: 10.1016/j.cmet.2017.10.008. Epub 2017 Nov 16. Cell Metab. 2018. PMID: 29153407 Free PMC article. - Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c.
Ferré P, Foufelle F. Ferré P, et al. Diabetes Obes Metab. 2010 Oct;12 Suppl 2:83-92. doi: 10.1111/j.1463-1326.2010.01275.x. Diabetes Obes Metab. 2010. PMID: 21029304 Review. - 3,5-diiodo-L-thyronine increases de novo lipogenesis in liver from hypothyroid rats by SREBP-1 and ChREBP-mediated transcriptional mechanisms.
Gnoni A, Siculella L, Paglialonga G, Damiano F, Giudetti AM. Gnoni A, et al. IUBMB Life. 2019 Jul;71(7):863-872. doi: 10.1002/iub.2014. Epub 2019 Feb 1. IUBMB Life. 2019. PMID: 30707786 - De novo lipogenesis in health and disease.
Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. Ameer F, et al. Metabolism. 2014 Jul;63(7):895-902. doi: 10.1016/j.metabol.2014.04.003. Epub 2014 Apr 12. Metabolism. 2014. PMID: 24814684 Review.
Cited by
- Manassantin B attenuates obesity by inhibiting adipogenesis and lipogenesis in an AMPK dependent manner.
Cai J, Qiong G, Li C, Sun L, Luo Y, Yuan S, Gonzalez FJ, Xu J. Cai J, et al. FASEB J. 2021 May;35(5):e21496. doi: 10.1096/fj.202002126RR. FASEB J. 2021. PMID: 33904622 Free PMC article. - Impact of Dysfunctional Adipose Tissue Depots on the Cardiovascular System.
D'Oria R, Genchi VA, Caccioppoli C, Calderoni I, Marrano N, Biondi G, Borrelli A, Di Gioia L, Giorgino F, Laviola L. D'Oria R, et al. Int J Mol Sci. 2022 Nov 18;23(22):14296. doi: 10.3390/ijms232214296. Int J Mol Sci. 2022. PMID: 36430774 Free PMC article. Review. - Maternal Fructose Intake, Programmed Mitochondrial Function and Predisposition to Adult Disease.
Smith EVL, Dyson RM, Weth FR, Berry MJ, Gray C. Smith EVL, et al. Int J Mol Sci. 2022 Oct 13;23(20):12215. doi: 10.3390/ijms232012215. Int J Mol Sci. 2022. PMID: 36293068 Free PMC article. Review. - Intrauterine food restriction impairs the lipogenesis process in the mesenteric adipocytes from low-birth-weight rats into adulthood.
Andreotti S, Komino ACM, de Fatima Silva F, Ramos APA, Gil NL, Azevedo GA, Sertié RAL, Lima FB, Landgraf RG, Landgraf MA. Andreotti S, et al. Front Endocrinol (Lausanne). 2023 Oct 31;14:1259854. doi: 10.3389/fendo.2023.1259854. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027196 Free PMC article. - Acetyl Co-A Carboxylase Inhibition Halts Hyperglycemia Induced Upregulation of De Novo Lipogenesis in Podocytes and Proximal Tubular Cells.
Kayampilly P, Roeser N, Rajendiran TM, Pennathur S, Afshinnia F. Kayampilly P, et al. Metabolites. 2022 Oct 3;12(10):940. doi: 10.3390/metabo12100940. Metabolites. 2022. PMID: 36295842 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous