Role of Neuroimaging on Differentiation of Parkinson's Disease and Its Related Diseases - PubMed (original) (raw)
Review
Role of Neuroimaging on Differentiation of Parkinson's Disease and Its Related Diseases
Toshihide Ogawa et al. Yonago Acta Med. 2018.
Abstract
An accurate diagnosis of Parkinson's disease (PD) is a prerequisite for therapeutic management. In spite of recent advances in the diagnosis of parkinsonian disorders, PD is misdiagnosed in between 6 and 25% of patients, even in specialized movement disorder centers. Although the gold standard for the diagnosis of PD is a neuropathological assessment, neuroimaging has been playing an important role in the differential diagnosis of PD and is used for clinical diagnostic criteria. In clinical practice, differential diagnoses of PD include atypical parkinsonian syndromes such as dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, caused by a striatal dopamine deficiency following nigrostrial degeneration. PD may also be mimicked by syndromes not associated with a striatal dopamine deficiency such as essential tremor, drug-induced parkinsonism, and vascular parkinsonism. Moreover, difficulties are associated with the clinical differentiation of patients with parkinsonism from those with Alzheimer's disease. In this review, we summarize the typical imaging findings of PD and its related diseases described above using morphological imaging modalities (conventional MR imaging and neuromelanin MR imaging) and functional imaging modalities (99mTc-ethyl cysteinate dimer perfusion single photon emission computed tomography, 123I-metaiodobenzylguanidine myocardial scintigraphy, and 123I-FP-CIT dopamine transporter single photon emission computed tomography) that are clinically available in most hospitals. We also attempt to provide a diagnostic approach for the differential diagnosis of PD and its related diseases in clinical practice.
Keywords: 123I-FP-CIT dopamine transporter imaging; 123I-metaiodobenzylguanidine myocardial scintigraphy; Parkinson’s disease; atypical parkinsonian syndrome; neuromelanin-sensitive MR imaging.
Figures
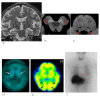
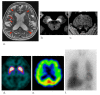
Fig. 1.
Parkinson’s disease in a 65-year-old female. (a) A T2-weighted MR image demonstrates no definite abnormalities including the midbrain. (b, c) NmMRI shows decreased hyperintensity in the lateral part of the substantia nigra (arrowheads) and locus coeruleus (arrowheads). (d) Markedly decreased striatal DaT uptake (arrows) is observed, particularly on the right side. (e) Perfusion SPECT shows mild hypoperfusion of the right basal ganglia (arrow). (f) Myocardial MIBG uptake (arrow) is markedly decreased. DaT, dopamine transporter; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
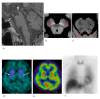
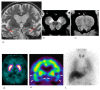
Fig. 2.
Dementia with Lewy bodies in an 88-year-old male. (a) A T2-weighted MR image demonstrates marked atrophy of the medial temporal lobes including the hippocampus. (b, c) NmMRI shows decreased hyperintensity in the lateral part of the substantia nigra (arrowheads) and locus coeruleus (arrowheads). (d) Markedly decreased striatal DaT uptake (arrows) is observed. (e) Perfusion SPECT shows bilateral occipital hypoperfusion (arrowheads) that is frequently observed in DLB. (f) Markedly decreased myocardial MIBG uptake (arrows) is also observed. DaT, dopamine transporter; DLB, dementia with Lewy bodies; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
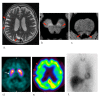
Fig. 3.
Multiple system atrophy in a 64-year-old female. (a) A T2-weighted MR image demonstrates cruciform hyperintensity (arrow) in the pons, termed the“hot cross bun.” (b, c) NmMRI shows decreased hyperintensity in the substantia nigra (arrowheads) and locus coeruleus (arrowheads). (d) Markedly decreased striatal DaT uptake (arrows) is observed. (e) Perfusion SPECT shows marked hypoperfusion (arrowheads) in the pons and cerebellum. (f) Myocardial MIBG scintigraphy shows normal myocardial MIBG uptake. DaT, dopamine transporter; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
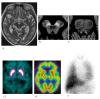
Fig. 4.
Progressive supranuclear palsy in a 71-year-old male. (a) A sagittal T1-weighted MR image shows midbrain atrophy with a concave (arrowhead) upper surface (hummingbird sign) compatible with PSP. (b, c) Decreased hyperintensity in the lateral substantia nigra (arrowheads) and locus coeruleus (arrowheads) is observed on NmMRI. (d) Decreased DaT uptake in the bilateral putamina (arrows) is observed. (e) Perfusion SPECT shows normal perfusion in both basal ganglia. (f) Myocardial MIBG scintigraphy shows normal myocardial MIBG uptake. DaT, dopamine transporter; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; PSP, progressive supranuclear palsy; SPECT, single photon emission computed tomography.
Fig. 5.
Corticobasal degeneration in a 79-year-old female. (a) A T2-weighted MR image demonstrates diffuse brain atrophy. Widening of the right frontal cortical sulci and parietooccipital sulcus are prominent (arrowheads). (b, c) NmMRI shows decreased hyperintensity in the lateral part of the substantia nigra (arrowheads) and locus coeruleus (arrowheads). (d) Slightly decreased striatal DaT uptakes (arrow) are observed in the right side. (e) Perfusion SPECT shows diffuse hypoperfusion of the cerebral cortex, particularly the right frontal lobe (arrowheads) (f) Myocardial MIBG uptake is preserved. DaT, dopamine transporter; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
Fig. 6.
Essential tremor in an 87-year-old female. (a) A T2-weighted MR image demonstrates no definite abnormalities in the midbrain. (b, c) Hyperintense regions in the substantia nigra and locus coeruleus are preserved on NmMRI. (d) DaTSCAN shows normal DaT uptake in the bilateral striatum. (e) Perfusion SPECT shows normal perfusion in the cerebral cortices and basal ganglia. (f) Myocardial MIBG scintigraphy shows normal myocardial MIBG uptake. DaT, dopamine transporter; DaTSCAN, dopamine transporter SPECT; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
Fig. 7.
Vascular parkinsonism in an 81-year-old female. (a) A T2-weighted MR image demonstrates small infarcts in the right basal ganglia (arrows) and diffuse white matter lesions (arrowheads). (b, c) Hyperintense regions in the substantia nigra and locus coeruleus are preserved on NmMRI. (d) DaTSCAN shows normal DaT uptake in the bilateral striatum. (e) Perfusion SPECT shows decreased hypoperfusion of the bilateral frontal cortices. (f) Myocardial MIBG scintigraphy shows normal myocardial MIBG uptake. DaT, dopamine transporter; DaTSCAN, dopamine transporter SPECT; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
Fig. 8.
Alzheimer’s disease in an 80-year-old male. (a) A T2-weighted MR image demonstrates marked atrophy of the bilateral temporal lobes including the hippocampus (arrows). (b, c) Hyperintense regions in the substantia nigra and locus coeruleus are preserved on NmMRI. (d) DaTSCAN shows normal DaT uptake in the bilateral striatum. (e) Perfusion SPECT shows marked hypoperfusion in the bilateral temporal lobes (arrowheads). (f) There are no abnormalities on myocardial MIBG scintigraphy. DaT, dopamine transporter; DaTSCAN, dopamine transporter SPECT; MIBG, 123I-metaiodobenzylguanidine; NmMRI, neuromelanin-sensitive MR imaging; SPECT, single photon emission computed tomography.
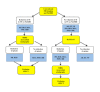
Fig. 9.
A diagnostic algorithm using neuroimaging for PD and its related diseases. AD, Alzheimer’s disease; CBD, corticobasal degeneration; DaTSCAN, dopamine transporter SPECT; DLB, dementia with Lewy bodies; ET, essential tremor; MIBG, 123I-metaiodobenzylguanidine; MSA, multiple system atrophy; NmMRI, neuromelanin-sensitive MR imaging; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; SI, signal intensity; SN, substantia nigra; SPECT; single photon emission computed tomography; VP, vascular parkinsonism.
Similar articles
- The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration.
Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. Cummings JL, et al. Brain. 2011 Nov;134(Pt 11):3146-66. doi: 10.1093/brain/awr177. Epub 2011 Aug 2. Brain. 2011. PMID: 21810889 Review. - Progression of dopaminergic degeneration in Parkinson's disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study.
Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffmann M, Brücke T. Pirker W, et al. Mov Disord. 2002 Jan;17(1):45-53. doi: 10.1002/mds.1265. Mov Disord. 2002. PMID: 11835438 - Neuroimaging in Parkinsonian Disorders.
Tripathi M, Kumar A, Bal C. Tripathi M, et al. Neurol India. 2018 Mar-Apr;66(Supplement):S68-S78. doi: 10.4103/0028-3886.226460. Neurol India. 2018. PMID: 29503329 Review. - Usefulness of combining 123I-FP-CIT-SPECT striatal asymmetry index and cardiac 123I-metaiodobenzylguanidine scintigraphy examinations for diagnosis of parkinsonisms.
Niimi Y, Ito S, Murate K, Hirota S, Hikichi C, Ishikawa T, Maeda T, Nagao R, Shima S, Mizutani Y, Ueda A, Mutoh T. Niimi Y, et al. J Neurol Sci. 2017 Jun 15;377:174-178. doi: 10.1016/j.jns.2017.04.026. Epub 2017 Apr 17. J Neurol Sci. 2017. PMID: 28477690 - Neuroimaging in Parkinson's Disease: necessity or exaggeration?
Śmiłowska K, Burzyńska-Makuch M, Brockhuis B, Piekarski R, Friedman A, Popek A, Sławek J. Śmiłowska K, et al. Neurol Neurochir Pol. 2021;55(6):536-548. doi: 10.5603/PJNNS.a2021.0068. Epub 2021 Oct 12. Neurol Neurochir Pol. 2021. PMID: 34637136
Cited by
- Emerging targets for the diagnosis of Parkinson's disease: examination of systemic biomarkers.
Cheslow L, Snook AE, Waldman SA. Cheslow L, et al. Biomark Med. 2021 Jun;15(8):597-608. doi: 10.2217/bmm-2020-0654. Epub 2021 May 14. Biomark Med. 2021. PMID: 33988462 Free PMC article. Review. - Standardization and Clinical Use of a Single-vial Formulation of Technetium-99m-Trodat Using Autoclave Method.
Thakur R, Nagaraj C, Joshi RK, Saini J, Yadav R, Kumar P. Thakur R, et al. Indian J Nucl Med. 2024 Jan-Feb;39(1):18-23. doi: 10.4103/ijnm.ijnm_104_23. Epub 2024 Mar 27. Indian J Nucl Med. 2024. PMID: 38817725 Free PMC article. - Elucidating the Role of Santalol as a Potent Inhibitor of Tyrosinase: In Vitro and In Silico Approaches.
Ali N, Zehra Z, Shamsi A, Beg MA, Parray ZA, Israil, Imam MA, Gaur NA, Hassan MI, Chaudhary AA, Rudayni HA, Alghonaim MI, Alsalamah SA, Islam A. Ali N, et al. Molecules. 2022 Dec 15;27(24):8915. doi: 10.3390/molecules27248915. Molecules. 2022. PMID: 36558055 Free PMC article. - The Significance of Vascular Pathogenesis in the Examination of Corticobasal Syndrome.
Dunalska A, Pikul J, Schok K, Wiejak KA, Alster P. Dunalska A, et al. Front Aging Neurosci. 2021 May 4;13:668614. doi: 10.3389/fnagi.2021.668614. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34017244 Free PMC article. Review. - Improved Performance of a Multipinhole SPECT for DAT Imaging by Increasing Number of Pinholes at the Expense of Increased Multiplexing.
Könik A, Zeraatkar N, Kalluri KS, Auer B, Fromme TJ, He Y, May M, Furenlid LR, Kuo PH, King MA. Könik A, et al. IEEE Trans Radiat Plasma Med Sci. 2021 Nov;5(6):817-825. doi: 10.1109/trpms.2020.3035626. Epub 2020 Nov 3. IEEE Trans Radiat Plasma Med Sci. 2021. PMID: 34746540 Free PMC article.
References
- Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, et al. EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol. 2013;20:16-34. - PubMed
- Rizzo G, Copetti M, Arcuti S, Martino S, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson’s disease. a systematic review and meta-analysis. Neurology. 2016;86:566-76. - PubMed
- Brooks DJ. Parkinson’s disease: diagnosis. Parkinsonism Relat Disord. 2012;Supp1:S31-33. - PubMed
Publication types
LinkOut - more resources
Full Text Sources