Theta Phase-Coordinated Memory Reactivation Reoccurs in a Slow-Oscillatory Rhythm during NREM Sleep - PubMed (original) (raw)
Theta Phase-Coordinated Memory Reactivation Reoccurs in a Slow-Oscillatory Rhythm during NREM Sleep
Thomas Schreiner et al. Cell Rep. 2018.
Abstract
It has been proposed that sleep's contribution to memory consolidation is to reactivate prior encoded information. To elucidate the neural mechanisms carrying reactivation-related mnemonic information, we investigated whether content-specific memory signatures associated with memory reactivation during wakefulness reoccur during subsequent sleep. We show that theta oscillations orchestrate the reactivation of memories during both wakefulness and sleep. Reactivation patterns during sleep autonomously re-emerged at a rate of ∼1 Hz, indicating a coordination by slow oscillatory activity.
Keywords: EEG; consolidation; episodic memory; memory reactivation; oscillations; phase similarity; retrieval; sleep; theta.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
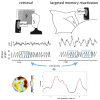
Experimental Design and Behavioral Results (A) Participants performed a vocabulary-learning task in the evening. They learned to associate Dutch words (cues) with German words (targets). After the initial learning phase, a cued recall, including feedback, was performed (recall1). Afterward, the cued recall was repeated without feedback (recall2). Subsequently, participants slept for 3 hr. During NREM sleep, 80 Dutch words (40 cued and 40 cued + feedback) were repeatedly presented. Memory performance was assessed in the final retrieval phase after sleep (B) Presenting single Dutch word cues during NREM sleep enhanced memory performance as compared to word-pair TMR and uncued words. Retrieval performance is indicated as percentage of recalled words, with performance before sleep set to 100%. Values are mean ± SEM. ∗∗p < 0.01.
Figure 2
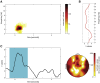
Word-Specific Phase Similarity during Wake Retrieval (A) Significantly enhanced phase similarity during successful subsequent retrieval was observed early after cue onset (t = 0 s) in the theta range. t-values were summed across electrodes in the significant cluster. (B) t-statistics of similarity results averaged over time and electrodes indicate a peak at 5 Hz. (C) Time course and topography of phase similarity at 5 Hz, indicating a rapid reactivation of memory content. The one-second time window around the center of the strongest cluster is highlighted. For the time course, t-values were averaged across all electrodes (n = 83), showing the content-specific phase-similarity effect. The topography displays summed t-values of the averaged difference between 0 and 2.5 s. See also Figures S1, S2, and S3.
Figure 3
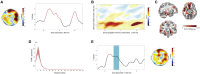
Word-Specific Phase Similarity between recall2 and TMR (A) Recurrent reactivation of recall-related phase patterns at 5 Hz during TMR emerged over right temporal electrodes. The topography displays the test statistics of the averaged difference in phase similarity between remembered and not-remembered words (0–2.5 s). The time course depicts t-values averaged across highlighted electrodes (n = 6). The phase similarity at a given time point reflects the similarity computed in a window of ±500 ms around this time point. (B) Assessing phase similarity at 5 Hz between every time point of retrieval and TMR confirmed the re-occurring pattern of similarity. (C) Source reconstruction. The difference in phase similarity for remembered and not-remembered items indicates effects in right (para)hippocampal regions and left frontal areas. (D) Frequency spectrum of the TMR similarity measures showed a ∼1 Hz periodicity of reactivation processes. Shading denotes SEM. (E) In line with behavioral predictions, providing a target stimulus after the TMR cue blocked associated reactivation processes. The time course depicts t-values averaged across highlighted electrodes in (A). Presentation of the target word is highlighted in petrol blue. Only a brief reactivation effect at 270 ms (before target word onset) emerged. The topography displays the test statistics of the averaged difference in phase similarity between remembered and not-remembered words (0–2.5 s). No significant cluster was found. See also Figures S1, S2, and S3.
Similar articles
- Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep.
Lehmann M, Schreiner T, Seifritz E, Rasch B. Lehmann M, et al. Sci Rep. 2016 Dec 16;6:39229. doi: 10.1038/srep39229. Sci Rep. 2016. PMID: 27982120 Free PMC article. - Sleep in Humans Stabilizes Pattern Separation Performance.
Hanert A, Weber FD, Pedersen A, Born J, Bartsch T. Hanert A, et al. J Neurosci. 2017 Dec 13;37(50):12238-12246. doi: 10.1523/JNEUROSCI.1189-17.2017. Epub 2017 Nov 8. J Neurosci. 2017. PMID: 29118106 Free PMC article. - Hippocampus-retrosplenial cortex interaction is increased during phasic REM and contributes to memory consolidation.
de Almeida-Filho DG, Koike BDV, Billwiller F, Farias KS, de Sales IRP, Luppi PH, Ribeiro S, Queiroz CM. de Almeida-Filho DG, et al. Sci Rep. 2021 Jun 22;11(1):13078. doi: 10.1038/s41598-021-91659-5. Sci Rep. 2021. PMID: 34158548 Free PMC article. - The memory function of sleep.
Diekelmann S, Born J. Diekelmann S, et al. Nat Rev Neurosci. 2010 Feb;11(2):114-26. doi: 10.1038/nrn2762. Epub 2010 Jan 4. Nat Rev Neurosci. 2010. PMID: 20046194 Review. - The role of the hippocampus in the consolidation of emotional memories during sleep.
Pronier É, Morici JF, Girardeau G. Pronier É, et al. Trends Neurosci. 2023 Nov;46(11):912-925. doi: 10.1016/j.tins.2023.08.003. Epub 2023 Sep 14. Trends Neurosci. 2023. PMID: 37714808 Review.
Cited by
- Neural Reinstatement of Overlapping Memories in Young and Older Adults.
Lee K, Mirjalili S, Quadri A, Corbett B, Duarte A. Lee K, et al. J Cogn Neurosci. 2022 Jul 1;34(8):1376-1396. doi: 10.1162/jocn_a_01871. J Cogn Neurosci. 2022. PMID: 35604351 Free PMC article. - Sleep spindles mediate hippocampal-neocortical coupling during long-duration ripples.
Ngo HV, Fell J, Staresina B. Ngo HV, et al. Elife. 2020 Jul 13;9:e57011. doi: 10.7554/eLife.57011. Elife. 2020. PMID: 32657268 Free PMC article. - Item-specific neural representations during human sleep support long-term memory.
Liu J, Xia T, Chen D, Yao Z, Zhu M, Antony JW, Lee TMC, Hu X. Liu J, et al. PLoS Biol. 2023 Nov 20;21(11):e3002399. doi: 10.1371/journal.pbio.3002399. eCollection 2023 Nov. PLoS Biol. 2023. PMID: 37983253 Free PMC article. - Heterogeneous profiles of coupled sleep oscillations in human hippocampus.
Cox R, Rüber T, Staresina BP, Fell J. Cox R, et al. Neuroimage. 2019 Nov 15;202:116178. doi: 10.1016/j.neuroimage.2019.116178. Epub 2019 Sep 7. Neuroimage. 2019. PMID: 31505272 Free PMC article. - The effect of zolpidem on targeted memory reactivation during sleep.
Carbone J, Bibián C, Reischl P, Born J, Forcato C, Diekelmann S. Carbone J, et al. Learn Mem. 2021 Aug 16;28(9):307-318. doi: 10.1101/lm.052787.120. Print 2021 Sep. Learn Mem. 2021. PMID: 34400532 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical