Initial treatment of steroid-sensitive idiopathic nephrotic syndrome in children with mycophenolate mofetil versus prednisone: protocol for a randomised, controlled, multicentre trial (INTENT study) - PubMed (original) (raw)
. 2018 Oct 10;8(10):e024882.
doi: 10.1136/bmjopen-2018-024882.
Marcus R Benz # 1, Jorg Doetsch 1, Alexander Fichtner 2, Jutta Gellermann 3, Dieter Haffner 4, Britta Höcker 2, Peter F Hoyer 5, Bärbel Kästner 6, Markus J Kemper 7, Martin Konrad 8, Steffen Luntz 6, Uwe Querfeld 3, Anja Sander 9, Burkhard Toenshoff # 2, Lutz T Weber # 1; Gesellschaft für Pädiatrische Nephrologie (GPN)
Affiliations
- PMID: 30309995
- PMCID: PMC6252704
- DOI: 10.1136/bmjopen-2018-024882
Initial treatment of steroid-sensitive idiopathic nephrotic syndrome in children with mycophenolate mofetil versus prednisone: protocol for a randomised, controlled, multicentre trial (INTENT study)
Rasmus Ehren et al. BMJ Open. 2018.
Abstract
Introduction: Idiopathic nephrotic syndrome is the most common glomerular disease in childhood with an incidence of 1.8 cases per 100 000 children in Germany. The treatment of the first episode implies two aspects: induction of remission and sustainment of remission. The recent Kidney Disease Improving Global Outcomes, American Academy of Pediatrics and German guidelines for the initial treatment of the first episode of a nephrotic syndrome recommend a 12-week course of prednisone. Despite being effective, this treatment is associated with pronounced glucocorticoid-associated toxicity due to high-dose prednisone administration over a prolonged period of time. The aim of the INTENT study (Initial treatment of steroid-sensitive idiopathic nephrotic syndrom in children with mycophenolate mofetil versus prednisone: protocol for a randomised, controlled, multicentre trial) is to show that an alternative treatment regimen with mycophenolic acid is not inferior regarding sustainment of remission, but with lower toxicity compared with treatment with glucocorticoids only.
Methods and design: The study is designed as an open, randomised, controlled, multicentre trial. 340 children with a first episode of steroid-sensitive nephrotic syndrome and who achieved remission by a standard prednisone regimen will be enrolled in the trial and randomised to one of two treatment arms. The standard care group will be treated with prednisone for a total of 12 weeks; in the experimental group the treatment is switched to mycophenolate mofetil, also for a total of 12 weeks in treatment duration. The primary endpoint is the occurrence of a treated relapse within 24 months after completion of initial treatment.
Ethics and dissemination: Ethics approval for this trial was granted by the ethics committee of the Medical Faculty of the University of Heidelberg (AFmu-554/2014). The study results will be published in accordance with the Consolidated Standards of Reporting Trials statement and the Standard Protocol Items: Recommendations for Interventional Trials guidelines. Our findings will be submitted to major international paediatric nephrology and general paediatric conferences and submitted for publication in a peer-reviewed, open-access journal.
Trial registration number: DRKS0006547; EudraCT2014-001991-76; Pre-result.
Date of registration: 30 October 2014; 24 February 2017.
Keywords: alternative treatment; mycophenolate mofetil; steroid-sensitive nephrotic syndrome; steroids.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RE, MRB, JD, AF, JG, DH, BH, PFH, BK, MJK, MK, SL, UQ and AS declare to have no competing interests. BT and LTW have received research grants from Roche Pharma AG and Novartis AG.
Figures
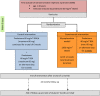
Figure 1
Trial schema. On alternate days means every second day. BSA, body surface area.
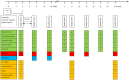
Figure 2
Study visit schedule. *Only in the experimental group. ABPM, ambulatory blood pressure monitoring; HRQoL, health-related quality of life; TDM MPA, therapeutic drug monitoring of mycophenolic acid.
Similar articles
- Interventions for idiopathic steroid-resistant nephrotic syndrome in children.
Hodson EM, Wong SC, Willis NS, Craig JC. Hodson EM, et al. Cochrane Database Syst Rev. 2016 Oct 11;10(10):CD003594. doi: 10.1002/14651858.CD003594.pub5. Cochrane Database Syst Rev. 2016. PMID: 27726125 Free PMC article. Updated. Review. - [Mycophenolate mofetil versus cyclosporine A in children with primary refractory nephrotic syndrome].
Geng HY, Ji LN, Chen CY, Tu J, Li HR, Bao R, Lin Y. Geng HY, et al. Zhonghua Er Ke Za Zhi. 2018 Sep 2;56(9):651-656. doi: 10.3760/cma.j.issn.0578-1310.2018.09.004. Zhonghua Er Ke Za Zhi. 2018. PMID: 30180402 Clinical Trial. Chinese. - A comparison of a standard-dose prednisone regimen and mycophenolate mofetil combined with a lower prednisone dose in Chinese adults with idiopathic nephrotic syndrome who were carriers of hepatitis B surface antigen: a prospective cohort study.
Li X, Tian J, Wu J, He Q, Li H, Han F, Li Q, Chen Y, Ni Q, Chen J. Li X, et al. Clin Ther. 2009 Apr;31(4):741-50. doi: 10.1016/j.clinthera.2009.04.011. Clin Ther. 2009. PMID: 19446147 Clinical Trial. - Study protocol: mycophenolate mofetil as maintenance therapy after rituximab treatment for childhood-onset, complicated, frequently-relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicenter double-blind, randomized, placebo-controlled trial (JSKDC07).
Horinouchi T, Sako M, Nakanishi K, Ishikura K, Ito S, Nakamura H, Oba MS, Nozu K, Iijima K. Horinouchi T, et al. BMC Nephrol. 2018 Nov 1;19(1):302. doi: 10.1186/s12882-018-1099-7. BMC Nephrol. 2018. PMID: 30382824 Free PMC article. Clinical Trial. - Steroid-sensitive nephrotic syndrome: an evidence-based update of immunosuppressive treatment in children.
Larkins N, Kim S, Craig J, Hodson E. Larkins N, et al. Arch Dis Child. 2016 Apr;101(4):404-8. doi: 10.1136/archdischild-2015-308924. Epub 2015 Aug 19. Arch Dis Child. 2016. PMID: 26289063 Review.
Cited by
- Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children.
Larkins NG, Hahn D, Liu ID, Willis NS, Craig JC, Hodson EM. Larkins NG, et al. Cochrane Database Syst Rev. 2024 Nov 8;11(11):CD002290. doi: 10.1002/14651858.CD002290.pub6. Cochrane Database Syst Rev. 2024. PMID: 39513526 Review. - Mycophenolate Mofetil Versus Prednisone for Induction Therapy in Steroid-Sensitive Idiopathic Nephrotic Syndrome in Children: An Observational Study.
Mazo A, Kilduff S, Pereira T, Solomon S, Matloff R, Zolotnitskaya A, Samsonov D. Mazo A, et al. Kidney Med. 2023 Dec 10;6(3):100776. doi: 10.1016/j.xkme.2023.100776. eCollection 2024 Mar. Kidney Med. 2023. PMID: 38435073 Free PMC article. - The effect of mycophenolate mofetil on podocytes in nephrotoxic serum nephritis.
Hackl A, Nüsken E, Voggel J, Abo Zed SED, Binz-Lotter J, Unnersjö-Jess D, Müller C, Fink G, Bohl K, Wiesner E, Diefenhardt P, Dafinger C, Chen H, Wohlfarth M, Müller RU, Hackl MJ, Schermer B, Nüsken KD, Weber LT. Hackl A, et al. Sci Rep. 2023 Aug 29;13(1):14167. doi: 10.1038/s41598-023-41222-1. Sci Rep. 2023. PMID: 37644089 Free PMC article. - Pediatric idiopathic steroid-sensitive nephrotic syndrome: diagnosis and therapy -short version of the updated German best practice guideline (S2e) - AWMF register no. 166-001, 6/2020.
Ehren R, Benz MR, Brinkkötter PT, Dötsch J, Eberl WR, Gellermann J, Hoyer PF, Jordans I, Kamrath C, Kemper MJ, Latta K, Müller D, Oh J, Tönshoff B, Weber S, Weber LT; German Society for Pediatric Nephrology. Ehren R, et al. Pediatr Nephrol. 2021 Oct;36(10):2971-2985. doi: 10.1007/s00467-021-05135-3. Epub 2021 Jun 6. Pediatr Nephrol. 2021. PMID: 34091756 Free PMC article. Review. - Steroid treatment for the first episode of childhood nephrotic syndrome: comparison of the 8 and 12 weeks regimen using an individual patient data meta-analysis.
Schijvens AM, Teeninga N, Dorresteijn EM, Teerenstra S, Webb NJ, Schreuder MF. Schijvens AM, et al. Eur J Pediatr. 2021 Sep;180(9):2849-2859. doi: 10.1007/s00431-021-04035-w. Epub 2021 Mar 28. Eur J Pediatr. 2021. PMID: 33774744 Free PMC article.
References
- Lawrenz C, 2010. Inzidenzanalyse des nephrotischen Syndroms im Kindes- und Jugendalter in den Jahren 2005–2006. Inaugural-Dissertation. Rheinische Friedrich-Wilhelm-Universität Bonn http://hss.ulb.uni-bonn.de/2010/2170/2170.pdf.
- Anon. Alternate-day prednisone is more effective than intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 1981;135:229–37. - PubMed
- Anon. Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Lancet 1988;1:380–3. - PubMed
- Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 1993;152:357–61. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials