Myogenin promotes myocyte fusion to balance fibre number and size - PubMed (original) (raw)
Myogenin promotes myocyte fusion to balance fibre number and size
Massimo Ganassi et al. Nat Commun. 2018.
Abstract
Each skeletal muscle acquires its unique size before birth, when terminally differentiating myocytes fuse to form a defined number of multinucleated myofibres. Although mice in which the transcription factor Myogenin is mutated lack most myogenesis and die perinatally, a specific cell biological role for Myogenin has remained elusive. Here we report that loss of function of zebrafish myog prevents formation of almost all multinucleated muscle fibres. A second, Myogenin-independent, fusion pathway in the deep myotome requires Hedgehog signalling. Lack of Myogenin does not prevent terminal differentiation; the smaller myotome has a normal number of myocytes forming more mononuclear, thin, albeit functional, fast muscle fibres. Mechanistically, Myogenin binds to the myomaker promoter and is required for expression of myomaker and other genes essential for myocyte fusion. Adult myog mutants display reduced muscle mass, decreased fibre size and nucleation. Adult-derived myog mutant myocytes show persistent defective fusion ex vivo. Myogenin is therefore essential for muscle homeostasis, regulating myocyte fusion to determine both muscle fibre number and size.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
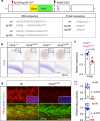
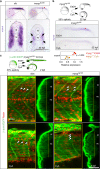
Genome editing generates zebrafish myog null alleles. a Schematic of myog gene exons (boxes) and protein showing the position of kg125, kg128 and fh265 mutant alleles. The tyrosine to stop mutations (Y37*) produce a truncated protein of 36 amino acids (aa) devoid of both basic (yellow) and helix–loop–helix (HLH, green) domains. The fh265 hypomorphic allele (Q167*) truncates downstream of bHLH. Beneath, DNA and protein alignment of wild-type (wt), 3 bp kg125 deletion (red) and 1 bp kg128 insertion (green) alleles with novel stop codons underlined causing an identical truncation. b In situ mRNA hybridisation (ISH) for myogenin on myog kg128/+ and myog kg125/+ incross lays reveal NMD of mutant myog mRNA at 20 somite stage (20 ss). Representative images n = 14 + 5 mutants, n = 39 + 22 sibs, respectively. Insets show magnification of boxed areas. c qPCR analysis on wt sibs and myog kg125 embryos at 22 ss confirms NMD. Mean fold change ± SEM from four independent experiments on genotyped embryos from four separate lays analysed on separate days, paired t test statistic. Symbol shapes denote matched wt and mutant samples from each experiment. d Immunoreactivity of Myog is lost in myog kg125 and myog kg128 mutants at 20 ss, whereas F-actin is unaffected. Insets show nuclear staining in myog kg125 and sib using Hoechst counterstain. Relative myotomal Myog immunofluorescence was assessed by nuclear intensity measurement. All images are lateral views anterior to left, dorsal to top. Representative images n = 10 + 7 mutants, n = 9 + 19 sibs, respectively. Bars = 50 µm
Fig. 2
Functional muscle differentiation in Myogenin mutants. a qPCR analysis on wt sibs and myog kg125 embryos at 22 ss showing reduction of myf5, mrf4 but not myod RNAs. Mean fold change ± SEM from four independent experiments on genotyped embryos from four separate lays analysed on separate days, paired t test statistic. Symbol shapes denote matched wt and myog mutant samples from each experiment. b, c Immunodetection of slow and fast myosins (sMyHC and fMyHC) in 2 dpf larvae from a myog kg125/+ incross showing that myofibre differentiation occurs in mutant. Dots in graphs show slow myofibre width (average of six myofibres/larva) and number in somite 17 of sib and mutant individuals. Representative images n = 5 mutants, n = 8 sibs. d–f Phalloidin staining for F-actin or immunolabelling for titin and α-actinin reveals that mutant myofibres display regular sarcomere spacing and are properly assembled to sustain contraction. Representative images n = 5 mutants, n = 21 sibs (phalloidin); n = 7 mutants, n = 18 sibs (titin); n = 7 mutants, n = 23 sibs (α-actinin). In f, boxes are shown magnified below. g Sarcomere length from f (average of 6 myofibres/larva) in sibs and mutants. Numbers of larvae analysed are shown on columns (b, g). h Larvae from a myog kg128/+ incross stained with α-bungarotoxin-Alexa-488 show that mutant embryos accumulate AChR at both neuromuscular junction (arrows) and muscle-muscle junction (arrowheads) comparable to sibs. Representative images n = 4 mutants, n = 11 sibs. i Motor function of 5 dpf larval zebrafish in fish-water (FW; n = 24 mutants, n = 115 sibs) or 0.6% Methyl-Cellulose (MC; n = 12 mutants, n = 36 sibs) as time spent moving (minutes), distance travelled (mm) and average speed (mm/s). Overall muscle function is unaffected by lack of Myog in both FW and MC. Activity of both myog mutants and sibs is affected by MC. Each dot represents the behaviour of an individual larva. ns: not statistically significant in ANOVA. Bars = 50 µm (10 µm in g)
Fig. 3
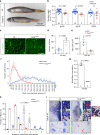
Myogenin is required for normal larval muscle growth. a, b ISH for mylpfa mRNA showing similar level of expression of fast myosin but reduced extent of somitic muscle in mutants (a, red brackets) at 1 and 2 dpf. Myotome height (b) is significantly reduced in myog kg128 mutants at 1 and 2 dpf. Representative images n = 9 + 18 mutants, n = 35 + 63 sibs. c Larval length is unaffected in mutant at 2 dpf, showing that muscle reduction is not due to overall reduced size of mutant larvae. d Schematic of myotome volume measurement. β-actin:EGFP;myog kg128/+ fish were incrossed and progeny imaged by confocal microscopy. Lateral and three equi-spaced transverse images of somite 17 were collected from each larva at each stage (dashed lines). Transverse area (red outline) multiplied by somite length (cyan dashed line) yielded myotome volume for each fish at each stage. e Optical transverse-sections of β-actin:EGFP;myog kg128 mutants show reduced myotome area at 2, 3 and 5 dpf compared to sibs (dashed red lines). Boxes are shown magnified beneath highlighting smaller fibre cross-sections in mutants (dashed white lines). Representative images n = 6 mutants, n = 14 sibs (2 dpf); n = 5 mutants, n = 18 sibs (3 dpf); n = 9 mutants, n = 13 sibs (5 dpf). f Myotome volume reduction in myog kg128 mutants at 2, 3 and 5 dpf. g–i. Myotome length (g), number of fast fibres per cross section (h) and fibre volume (i) at 2 dpf compared by t tests. Bars = 50 μm. nt: neural tube, nc: notochord
Fig. 4
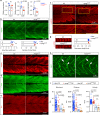
Myogenin promotes fusion of myocytes. a, b Immunodetection and quantification of Pax3/7 positive MPCs in somite 17 of myog kg125/+ incross embryos at 1 dpf and 2 dpf. Mean ± SEM of dots representing individual embryos. Lack of Myog does not alter number of Pax3/7 MPCs per somite (white dashed lines). Representative images n = 6 mutants, n = 11 sibs (1 dpf); n = 8 mutants, n = 9 sibs (2 dpf). c Qualitative analysis of myoblast fusion in a myog kg125/+ incross injected at 1-cell stage with DNA encoding CAAX-membrane targeted mCherry (red). At 2 dpf, larvae were fixed and stained with Hoechst to highlight nuclei (blue) and analysed from 3D stacks. Nuclei within mCherry-labelled fibres (arrowheads) were mostly single in mutants, but multiple in sibs. Representative images n = 6 embryos. d Myoblast fusion quantified by injection of H2B:mCherry RNA into 1-cell stage embryos from β-actin:EGFP;myog kg128/+ incross. Confocal single plane images deep in the myotome of 2 dpf larvae showing muscle fibres and the position of nuclei (insets). Note the central location away from somite borders (dashed white lines) of most nuclei in mutants (arrowheads), similar to that observed in mononucleate superficial slow fibres. Representative images n = 6 embryos. e, f Quantification of fusion within the entire myotome 17, showing the fraction of fast fibres (e) and fraction of nuclei in fast fibres (f) with the indicated number of nuclei. Slow fibre numbers were unaltered. Data report mean values of three larvae per genotype (see Supplementary Fig. 3a for individual data). _p_-values indicate probability of rejecting null hypothesis of no difference between mutant and sibs in _χ_2 tests. g Number of nuclei per fast fibre is reduced in mutant. h Total number of nuclei within fast fibres in somite 17 of sib and mutant is unchanged. Dots represent individual embryos. Mean ± SEM. t test. Bars = 50 μm. i, j Mosaic myog:MyogCDS-IRES-GFP plasmid-derived expression of Myog (Myog O/E) rescues fusion in myog kg128 mutant larvae from a myog kg128/+ incross, compared control myog:GFP plasmid (Control). Quantification (as in e) of nuclei in GFP+ cells (i, see Supplementary Fig. 4a for individual data). Immunodetection shows Myog overexpression (Myog O/E, magenta) in myog:MyogCDS-IRES-GFP but not in myog:GFP(Control, green) GFP+ fibres (j). Representative images n = 5 myog:GFP, n = 11 myog:MyogCDS-IRES-GFP injected mutants, ns: not significant
Fig. 5
Myogenin mutants have reduced expression of fusogenic factors. a ISH on 20 hpf myog kg128/+ incross to investigate the expression of genes essential for vertebrate myocyte fusion. Expression of myomaker (mymk) and jam3b, but not of jam2a or kirrel3l, is reduced in myog mutant. Bar = 50 μm. Representative images n = 10 mutants, n = 38 sibs (mymk); n = 13 mutants, n = 37 sibs (jam2a); n = 12 mutants, n = 39 sibs (jam3b); n = 7 mutants, n = 13 sibs (kirrel3l). b qPCR analysis of RNA expression levels showing downregulation of mymk, myomixer/myomerger/minion (mymx) and jam3b at 20 hpf on myog kg125 mutants, whereas jam2a and kirrel3l remain unaltered compared to wt (myog+/+) sibs. Graphs show mean fold change ± SEM of four independent experiments; paired t test. Symbol shapes denote wt and mutant samples from paired experiments. c Schematic of 5' genomic region of mymk reporting: 5′-UTR and coding sequence (grey and white boxes), position of E-box elements (red boxes), relative bp distance from 5'UTR start (+1), start codon (arrow, ATG). E-box 1 and E-box 2 sequences are shown in red text. d ChIP-qPCR assay using anti-Myog or mock showing significant Myogenin enrichment on mymk E-box 1 compared to negative controls from the gapdh promoter and a gene-free region on chromosome 14 containing an E-box (Chr14). Mean of percentage of input immunoprecipitated ± SD of two independent experiments, ANOVA
Fig. 6
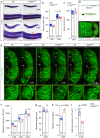
Hedgehog signalling sustains residual fusion and mymk expression. a ISH for myomaker (mymk) at 20 hpf revealed that residual expression in myog mutant is enriched in the medial region of the somite close to notochord (arrows in transverse sections from indicated axial level, dorsal to top). Note lack of expression in mononucleate slow pioneer fibres (arrowheads, upper panel). Representative images n = 6 mutants, n = 14 wt sibs (mymk) b ISH (lateral view, dorsal to top) and qPCR analysis showing that cyclopamine (CyA) treatment of myog kg125 embryos almost abolished mymk mRNA compared to ethanol (EtOH) vehicle control. CyA effectiveness is shown by the absence of unstained slow muscle pioneer cells (arrowhead). Mean fold change ± SEM from three independent experiments on embryos from separate lays of myog kg125 (circles) and myog kg128 (squares and triangles) analysed on separate days, paired t test statistic. Representative images n = 4 EtOH, n = 6 CyA. c Optical confocal sections of the medial region of somites 17 of β-actin:EGFP;myog kg128/+ incross treated with vehicle or CyA. Transverse-section panels show medial position (yellow lines) of respective longitudinal section for each condition. CyA abolished residual fusion in the medial myotome of mutant embryos (arrowheads) but did not detectably affect fusion in sibs. Note that the residual multinucleate fibres in myog kg125 mutant appear larger than adjacent mononucleate fibres in EtOH but are lacking in CyA. nt: neural tube, nc: notochord. Representative images n = 5 mutants, n = 3 sibs (EtOH); n = 4 mutants, n = 6 sibs (CyA). Bars = 50 μm
Fig. 7
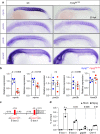
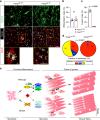
Adult Myogenin mutants have reduced muscle with more but smaller myofibres. a Myog mutant and sib at 120 dpf from myog kg128/+ incross. Bar = 1 cm. Representative images n = 5 mutants, n = 23 sibs. b Myog kg128 or myog kg125 but not myog _f_fh265 showed reduced standard weight compared to co-reared sibs at 120 dpf. Dots represent individuals. c Laminin immunodetection on cryosections from 120 dpf myog 128/+ incross. Bar = 100 µm. Representative images, n = 3. d–f Number of muscle fibres in 0.1 mm2 of adult muscle is increased in mutants (d), whereas myofibre cross-sectional area (CSA) is decreased (e) reflecting a shift in CSA frequency distribution compared to sibs. g Fewer myonuclear profiles were present within laminin profiles in adult muscle cross-sections in mutants than in sibs, measured from 107 to 490 fibres at similar medio-lateral and dorso-ventral positions of trunk muscle of three fish per genotype. Mean ± SEM, t test. h Proportions of muscle fibres with indicated number of myonuclei within fibre cross-sectional profile. In sibs, >90% of fibres have more than one nuclear profile, compared with < 15% in mutants. Mean ± SEM, _χ_2 test. i NADH tetrazolium reductase stain revealed that in both mutants and sibs three fibre types are present: oxidative/slow (slow), intermediate (int) and glycolytic/fast (fast). Size of more glycolytic myofibres (yellow and green insets) is more reduced than oxidative fibres (cyan). Assay was performed on three 120 dpf adult male length-matched fish of each genotype. Representative sib (blue) or mut (red) fibres are highlighted. Mutant presents smaller slow type myofibres ectopically localised in fast domain (red inset). Representative images, n = 3. Bars = 100 μm (except for red, yellow and cyan insets = 10 μm)
Fig. 8
Mutant adult-derived muscle progenitor cells retain fusion deficit ex vivo. a Immunodetection of desmin (green) and MyHC (red) and nuclei (white, Hoechst) in 15 months old myog kg125 and sibling myog kg125/+ adult-derived MPCs following 5 days of differentiation. Fusion into multinucleated myofibres occurred only in sib (magnified boxes), coloured arrowheads indicate nuclei of each cell. Representative images, n = 3. b Extent of differentiation (Differentiation index) is comparable between mutant and heterozygous MPCs. c Fusion index showing deficit in fusion of mutant myocytes. d Number of nuclei in fused MyHC+ cells is reduced in mutant, _χ_2 test. Three fish per genotype (three technical replicates each). e Schematic of the role of Myogenin during differentiation, fusion and growth of muscle fibres. During myogenesis, committed MPCs leave the cell cycle, begin to elongate and express early muscle-specific genes during terminal differentiation into myocytes. At this stage, Myogenin (MYOG) promotes the expression of myomaker (MYMK), myomixer (MYMX) and jam3b (JAM3B). These fusogenic proteins prompt myocyte fusion to form muscle fibres. In the absence of Myogenin, myocytes undergo terminal differentiation but fail to express Myog-module genes, remain mononucleated and grow less throughout life. Residual myocyte fusion in Myog mutants in the medial region of the somite (bracket) is sustained by Hedgehog (Hh) signalling
Similar articles
- Myomaker is required for the fusion of fast-twitch myocytes in the zebrafish embryo.
Zhang W, Roy S. Zhang W, et al. Dev Biol. 2017 Mar 1;423(1):24-33. doi: 10.1016/j.ydbio.2017.01.019. Epub 2017 Feb 1. Dev Biol. 2017. PMID: 28161523 - Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis.
Ganassi M, Badodi S, Wanders K, Zammit PS, Hughes SM. Ganassi M, et al. Elife. 2020 Oct 1;9:e60445. doi: 10.7554/eLife.60445. Elife. 2020. PMID: 33001028 Free PMC article. - Myogenin is required for assembly of the transcription machinery on muscle genes during skeletal muscle differentiation.
Adhikari A, Kim W, Davie J. Adhikari A, et al. PLoS One. 2021 Jan 19;16(1):e0245618. doi: 10.1371/journal.pone.0245618. eCollection 2021. PLoS One. 2021. PMID: 33465133 Free PMC article. - Control of muscle fibre-type diversity during embryonic development: the zebrafish paradigm.
Jackson HE, Ingham PW. Jackson HE, et al. Mech Dev. 2013 Sep-Oct;130(9-10):447-57. doi: 10.1016/j.mod.2013.06.001. Epub 2013 Jun 28. Mech Dev. 2013. PMID: 23811405 Review. - Molecular regulation of myocyte fusion.
Wherley TJ, Thomas S, Millay DP, Saunders T, Roy S. Wherley TJ, et al. Curr Top Dev Biol. 2024;158:53-82. doi: 10.1016/bs.ctdb.2024.01.016. Epub 2024 Mar 16. Curr Top Dev Biol. 2024. PMID: 38670716 Review.
Cited by
- Aerobic Exercise Protects against Cardiotoxin-Induced Skeletal Muscle Injury in a DDAH1-Dependent Manner.
Feng F, Luo K, Yuan X, Lan T, Wang S, Xu X, Lu Z. Feng F, et al. Antioxidants (Basel). 2024 Sep 1;13(9):1069. doi: 10.3390/antiox13091069. Antioxidants (Basel). 2024. PMID: 39334728 Free PMC article. - Sporadic inclusion body myositis-derived myotube culture revealed muscle cell-autonomous expression profiles.
Suzuki N, Kanzaki M, Koide M, Izumi R, Fujita R, Takahashi T, Ogawa K, Yabe Y, Tsuchiya M, Suzuki M, Harada R, Ohno A, Ono H, Nakamura N, Ikeda K, Warita H, Osana S, Oikawa Y, Toyohara T, Abe T, Rui M, Ebihara S, Nagatomi R, Hagiwara Y, Aoki M. Suzuki N, et al. PLoS One. 2024 Aug 1;19(8):e0306021. doi: 10.1371/journal.pone.0306021. eCollection 2024. PLoS One. 2024. PMID: 39088432 Free PMC article. - Skeletal muscle: molecular structure, myogenesis, biological functions, and diseases.
Feng LT, Chen ZN, Bian H. Feng LT, et al. MedComm (2020). 2024 Jul 10;5(7):e649. doi: 10.1002/mco2.649. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 38988494 Free PMC article. Review. - Lactiplantibacillus plantarum LM1001 Improves Digestibility of Branched-Chain Amino Acids in Whey Proteins and Promotes Myogenesis in C2C12 Myotubes.
Lee Y, So YJ, Jung WH, Kim TR, Sohn M, Jeong YJ, Imm JY. Lee Y, et al. Food Sci Anim Resour. 2024 Jul;44(4):951-965. doi: 10.5851/kosfa.2024.e38. Epub 2024 Jul 1. Food Sci Anim Resour. 2024. PMID: 38974720 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- G1001029/Medical Research Council (MRC)/International
- PG/14/12/30664/British Heart Foundation (BHF)/International
- PG/14/12/30664/BHF_/British Heart Foundation/United Kingdom
- MR/P023215/1/Medical Research Council (MRC)/International
- N021231/1/Medical Research Council (MRC)/International
- MR/P023215/1/MRC_/Medical Research Council/United Kingdom
- G1001029/MRC_/Medical Research Council/United Kingdom
- AFM17865/Association Française contre les Myopathies (AFM)/International
- MR/N021231/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases