Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease - PubMed (original) (raw)
Review
Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease
Tawfik Addi et al. Toxins (Basel). 2018.
Abstract
Patients with chronic kidney disease (CKD) display an elevated risk of thrombosis. Thrombosis occurs in cardiovascular events, such as venous thromboembolism, stroke, and acute coronary syndrome, and is a cause of hemodialysis vascular access dysfunction. CKD leads to the accumulation of uremic toxins, which exerts toxic effects on blood and the vessel wall. Some uremic toxins result from tryptophan metabolization in the gut through the indolic and the kynurenine pathways. An increasing number of studies are highlighting the link between such uremic toxins and thrombosis in CKD. In this review, we describe the thrombotic mechanisms induced by tryptophan-derived uremic toxins (TDUT). These mechanisms include an increase in plasma levels of procoagulant factors, induction of platelet hyperactivity, induction of endothelial dysfunction/ impairment of endothelial healing, decrease in nitric oxide (NO) bioavailability, and production of procoagulant microparticles. We focus on one important prothrombotic mechanism: The induction of tissue factor (TF), the initiator of the extrinsic pathway of the blood coagulation. This induction occurs via a new pathway, dependent on the transcription factor Aryl hydrocarbon receptor (AhR), the receptor of TDUT in cells. A better understanding of the prothrombotic mechanisms of uremic toxins could help to find novel therapeutic targets to prevent thrombosis in CKD.
Keywords: chronic kidney disease; thrombosis; tryptophan; uremic toxins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
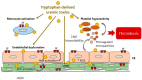
Figure 1
Mechanisms of tryptophan-derived uremic toxins promoting thrombosis. Tryptophan-derived uremic toxins promote thrombosis by inducing platelet hyperactivity, endothelial dysfunction, production of endothelial and platelet microparticles, and TF expression on monocytes, vSMC, and EC. TF induction is mediated by AhR activation. A decrease in NO bioavailability and an induction of ROS in platelets and endothelial cells participate in the thrombotic effects of tryptophan-derived uremic toxins. AhR: Aryl hydrocarbon Receptor; TF: Tissue Factor; EC: Endothelial cells, vSMC: Vascular smooth muscle cells; ROS: Reactive oxygen species; NO: Nitric Oxide.
Similar articles
- The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease.
Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. Sallée M, et al. Toxins (Basel). 2014 Mar 4;6(3):934-49. doi: 10.3390/toxins6030934. Toxins (Basel). 2014. PMID: 24599232 Free PMC article. Review. - Aryl Hydrocarbon Receptor Activation in Chronic Kidney Disease: Role of Uremic Toxins.
Brito JS, Borges NA, Esgalhado M, Magliano DC, Soulage CO, Mafra D. Brito JS, et al. Nephron. 2017;137(1):1-7. doi: 10.1159/000476074. Epub 2017 May 11. Nephron. 2017. PMID: 28490014 Review. - How do Uremic Toxins Affect the Endothelium?
Cunha RSD, Santos AF, Barreto FC, Stinghen AEM. Cunha RSD, et al. Toxins (Basel). 2020 Jun 20;12(6):412. doi: 10.3390/toxins12060412. Toxins (Basel). 2020. PMID: 32575762 Free PMC article. Review. - Thrombolome and Its Emerging Role in Chronic Kidney Diseases.
Fryc J, Naumnik B. Fryc J, et al. Toxins (Basel). 2021 Mar 18;13(3):223. doi: 10.3390/toxins13030223. Toxins (Basel). 2021. PMID: 33803899 Free PMC article. Review. - Effects of Uremic Toxins from the Gut Microbiota on Bone: A Brief Look at Chronic Kidney Disease.
Black AP, Cardozo LF, Mafra D. Black AP, et al. Ther Apher Dial. 2015 Oct;19(5):436-40. doi: 10.1111/1744-9987.12307. Epub 2015 May 5. Ther Apher Dial. 2015. PMID: 25944654 Review.
Cited by
- Tissue Factor, Thrombosis, and Chronic Kidney Disease.
Oe Y, Takahashi N. Oe Y, et al. Biomedicines. 2022 Oct 28;10(11):2737. doi: 10.3390/biomedicines10112737. Biomedicines. 2022. PMID: 36359257 Free PMC article. Review. - Breastfeeding and Future Cardiovascular, Kidney, and Metabolic Health-A Narrative Review.
Tain YL, Lin YJ, Hsu CN. Tain YL, et al. Nutrients. 2025 Mar 12;17(6):995. doi: 10.3390/nu17060995. Nutrients. 2025. PMID: 40290039 Free PMC article. Review. - Kynurenine Pathway in Chronic Kidney Disease: What's Old, What's New, and What's Next?
Mor A, Kalaska B, Pawlak D. Mor A, et al. Int J Tryptophan Res. 2020 Sep 21;13:1178646920954882. doi: 10.1177/1178646920954882. eCollection 2020. Int J Tryptophan Res. 2020. PMID: 35210786 Free PMC article. Review. - Indoxyl Sulfate, a Uremic Endotheliotoxin.
Lano G, Burtey S, Sallée M. Lano G, et al. Toxins (Basel). 2020 Apr 5;12(4):229. doi: 10.3390/toxins12040229. Toxins (Basel). 2020. PMID: 32260489 Free PMC article. Review. - Unveiling the role of sex in the metabolism of indoxyl sulfate and apixaban.
Pina-Beltran B, Dimitrov D, McKay N, Giot M, Zdráhal Z, Potěšil D, Pustka V, Peinado-Izaguerri J, Saez-Rodriguez J, Poitevin S, Burtey S. Pina-Beltran B, et al. Sci Rep. 2025 Feb 19;15(1):6075. doi: 10.1038/s41598-025-90405-5. Sci Rep. 2025. PMID: 39972038 Free PMC article.
References
- Levin A., Stevens P., Bilous R.W., Coresh J., De Francisco A.L., De Jong P.E., Griffi K.E., Hemmelgarn B.R., Isek K., Lamb E.J., et al. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013;3:19–62. doi: 10.1038/kisup.2012.64. - DOI
- Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. - DOI - PubMed
- Coresh J., Turin T.C., Matsushita K., Sang Y., Ballew S.H., Appel L.J., Arima H., Chadban S.J., Cirillo M., Djurdjev O., et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. - DOI - PMC - PubMed
- Herzog C.A., Asinger R.W., Berger A.K., Charytan D.M., Díez J., Hart R.G., Eckardt K.-U., Kasiske B.L., McCullough P.A., Passman R.S., et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous