Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome - PubMed (original) (raw)
doi: 10.1016/j.jalz.2018.07.217. Epub 2018 Oct 15.
Matthias Arnold 2, Kwangsik Nho 3, Shahzad Ahmad 4, Wei Jia 5, Guoxiang Xie 6, Gregory Louie 1, Alexandra Kueider-Paisley 1, M Arthur Moseley 7, J Will Thompson 7, Lisa St John Williams 7, Jessica D Tenenbaum 8, Colette Blach 9, Rebecca Baillie 10, Xianlin Han 11, Sudeepa Bhattacharyya 12, Jon B Toledo 13, Simon Schafferer 14, Sebastian Klein 14, Therese Koal 14, Shannon L Risacher 3, Mitchel Allan Kling 15, Alison Motsinger-Reif 16, Daniel M Rotroff 16, John Jack 16, Thomas Hankemeier 17, David A Bennett 18, Philip L De Jager 19, John Q Trojanowski 20, Leslie M Shaw 20, Michael W Weiner 21, P Murali Doraiswamy 22, Cornelia M van Duijn 4, Andrew J Saykin 23, Gabi Kastenmüller 24, Rima Kaddurah-Daouk 25; Alzheimer's Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium
Affiliations
- PMID: 30337151
- PMCID: PMC6487485
- DOI: 10.1016/j.jalz.2018.07.217
Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome
Siamak MahmoudianDehkordi et al. Alzheimers Dement. 2019 Jan.
Erratum in
- Erratum to "Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome" [Alzheimer's & Dementia 2019;15:76-92.].
[No authors listed] [No authors listed] Alzheimers Dement. 2019 Apr;15(4):604. doi: 10.1016/j.jalz.2019.03.002. Epub 2019 Mar 21. Alzheimers Dement. 2019. PMID: 30905591 Free PMC article. No abstract available.
Abstract
Introduction: Increasing evidence suggests a role for the gut microbiome in central nervous system disorders and a specific role for the gut-brain axis in neurodegeneration. Bile acids (BAs), products of cholesterol metabolism and clearance, are produced in the liver and are further metabolized by gut bacteria. They have major regulatory and signaling functions and seem dysregulated in Alzheimer's disease (AD).
Methods: Serum levels of 15 primary and secondary BAs and their conjugated forms were measured in 1464 subjects including 370 cognitively normal older adults, 284 with early mild cognitive impairment, 505 with late mild cognitive impairment, and 305 AD cases enrolled in the AD Neuroimaging Initiative. We assessed associations of BA profiles including selected ratios with diagnosis, cognition, and AD-related genetic variants, adjusting for confounders and multiple testing.
Results: In AD compared to cognitively normal older adults, we observed significantly lower serum concentrations of a primary BA (cholic acid [CA]) and increased levels of the bacterially produced, secondary BA, deoxycholic acid, and its glycine and taurine conjugated forms. An increased ratio of deoxycholic acid:CA, which reflects 7α-dehydroxylation of CA by gut bacteria, strongly associated with cognitive decline, a finding replicated in serum and brain samples in the Rush Religious Orders and Memory and Aging Project. Several genetic variants in immune response-related genes implicated in AD showed associations with BA profiles.
Discussion: We report for the first time an association between altered BA profile, genetic variants implicated in AD, and cognitive changes in disease using a large multicenter study. These findings warrant further investigation of gut dysbiosis and possible role of gut-liver-brain axis in the pathogenesis of AD.
Keywords: Alzheimer's disease; Atlas for Alzheimer; Genetic variants; Gut microbiome; Gut-liver-brain axis; Immunity; Inflammation; Lipidomics; Metabolome; Metabolomics.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Fig. 1. Bile acid synthesis and cholesterol clearance pathway.
Regulation of bile acid synthesis by feedback mechanism and bile acid transport through enterohepatic circulation. In the liver the bile acids (CDCA, DCA, LCA, CA) activate FXR that inhibits (via a repressor SHP, not shown here) the rate-limiting enzyme CYP7A1. The bile acids via FXR/SHP also inhibit the influx transporter NTCP; induce BSEP and canalicular bile acid secretion. In the intestine, bile acids, via FXR, inhibit the uptake transporter ASBT, decreasing absorption and increasing basolateral secretion into portal circulation by inducing OSTα & β. Bile acid activated FXR in the intestine also exerts inhibition on CYP7A1 in the liver via FGF19 pathway. At the basolateral membrane of hepatocytes, transporters OSTα & β, and also MRP3 and MRP4, secrete bile acids into the systemic circulation. Abbreviations: ASBT: Apical Sodium-dependent Bile acid Transporters; BSEP: Bile Salt Export Pump; FXR: Farnesoid X Receptor; NTCP: Sodium/Taurocholate Co-transporting Polypeptide; SHP: Small heterodimer partner.
Fig. 1. Bile acid synthesis and cholesterol clearance pathway.
Regulation of bile acid synthesis by feedback mechanism and bile acid transport through enterohepatic circulation. In the liver the bile acids (CDCA, DCA, LCA, CA) activate FXR that inhibits (via a repressor SHP, not shown here) the rate-limiting enzyme CYP7A1. The bile acids via FXR/SHP also inhibit the influx transporter NTCP; induce BSEP and canalicular bile acid secretion. In the intestine, bile acids, via FXR, inhibit the uptake transporter ASBT, decreasing absorption and increasing basolateral secretion into portal circulation by inducing OSTα & β. Bile acid activated FXR in the intestine also exerts inhibition on CYP7A1 in the liver via FGF19 pathway. At the basolateral membrane of hepatocytes, transporters OSTα & β, and also MRP3 and MRP4, secrete bile acids into the systemic circulation. Abbreviations: ASBT: Apical Sodium-dependent Bile acid Transporters; BSEP: Bile Salt Export Pump; FXR: Farnesoid X Receptor; NTCP: Sodium/Taurocholate Co-transporting Polypeptide; SHP: Small heterodimer partner.
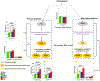
Fig.2.
Schematic representation of study design.
Fig. 3.
Ratios of bile acids reflective of liver and gut microbiome enzymatic activities in CN, Early MCI, Late MCI and AD patients. Three types of ratios were calculated to inform about possible enzymatic activity changes in Alzheimer’s patients. These ratios reflect one of the following: (1) Shift in bile acid metabolism from primary to alternative pathway. (2) Changes in gut microbiome correlated with production of secondary bile acids. (3) Changes in glycine and taurine conjugation of secondary bile acids. Color code: Green: cognitively normal; Yellow: EMCI; Blue: LMCI; Red: AD. Composition of selected ratios stratified by clinical diagnosis. Error bars indicate standard error of the means; Asterisks indicate statistical significance (*P<10−03, ** P< 10−04, and ***P< 10−05). _P_-values were estimated from logistic regression models and adjusted for age, sex, body mass index, and APOE ε4 status. The significance level was adjusted for multiple testing according to Bonferroni method to 0.05/138 = 3.62E-4; LCA was excluded in the quality control steps.
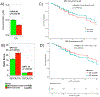
Fig. 4.
Comparison of bile levels in MCI subjects who convert and those who did not convert to AD dementia. A and B. Lower levels of CA and higher levels of two secondary to primary ratios were significantly associated with higher odds of converting from MCI to AD. EMCI and LMCI patients that converted to AD dementia in 4 years after baseline were labeled as MCI-Converter; 9 bile acids and ratios that were significantly dysregulated between CN to AD were assessed; _P_-values were estimated from logistic regression models and adjusted for age, sex, body mass index, and APOE ε4 status; the significance level was adjusted for multiple testing according to Bonferroni 0.05/9 = 5.56 × 10−3. C and D. Cox hazards model of the association of conversion from MCI to AD. Red line: 1st quantile, Red line: 3rd quantile. Analysis was conducted using quantitative values and stratification by quantiles was used only for graphical representation.
Similar articles
- Altered bile acid profile in mild cognitive impairment and Alzheimer's disease: Relationship to neuroimaging and CSF biomarkers.
Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, Jia W, Xie G, Ahmad S, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R; Alzheimer's Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium. Nho K, et al. Alzheimers Dement. 2019 Feb;15(2):232-244. doi: 10.1016/j.jalz.2018.08.012. Epub 2018 Oct 15. Alzheimers Dement. 2019. PMID: 30337152 Free PMC article. - Bile acid synthesis, modulation, and dementia: A metabolomic, transcriptomic, and pharmacoepidemiologic study.
Varma VR, Wang Y, An Y, Varma S, Bilgel M, Doshi J, Legido-Quigley C, Delgado JC, Oommen AM, Roberts JA, Wong DF, Davatzikos C, Resnick SM, Troncoso JC, Pletnikova O, O'Brien R, Hak E, Baak BN, Pfeiffer R, Baloni P, Mohmoudiandehkordi S, Nho K, Kaddurah-Daouk R, Bennett DA, Gadalla SM, Thambisetty M. Varma VR, et al. PLoS Med. 2021 May 27;18(5):e1003615. doi: 10.1371/journal.pmed.1003615. eCollection 2021 May. PLoS Med. 2021. PMID: 34043628 Free PMC article. - Compositional and functional gut microbiota alterations in mild cognitive impairment: links to Alzheimer's disease pathology.
Fan KC, Lin CC, Chiu YL, Koh SH, Liu YC, Chuang YF. Fan KC, et al. Alzheimers Res Ther. 2025 May 30;17(1):122. doi: 10.1186/s13195-025-01769-9. Alzheimers Res Ther. 2025. PMID: 40448221 Free PMC article. - Bile Acids as Key Modulators of the Brain-Gut-Microbiota Axis in Alzheimer's Disease.
Mulak A. Mulak A. J Alzheimers Dis. 2021;84(2):461-477. doi: 10.3233/JAD-210608. J Alzheimers Dis. 2021. PMID: 34569953 Free PMC article. Review. - Molecular mechanism and therapeutic strategy of bile acids in Alzheimer's disease from the emerging perspective of the microbiota-gut-brain axis.
Wu M, Cheng Y, Zhang R, Han W, Jiang H, Bi C, Zhang Z, Ye M, Lin X, Liu Z. Wu M, et al. Biomed Pharmacother. 2024 Sep;178:117228. doi: 10.1016/j.biopha.2024.117228. Epub 2024 Jul 31. Biomed Pharmacother. 2024. PMID: 39088965 Review.
Cited by
- Gut microbiota metabolites: potential therapeutic targets for Alzheimer's disease?
Zhang S, Lu J, Jin Z, Xu H, Zhang D, Chen J, Wang J. Zhang S, et al. Front Pharmacol. 2024 Sep 17;15:1459655. doi: 10.3389/fphar.2024.1459655. eCollection 2024. Front Pharmacol. 2024. PMID: 39355779 Free PMC article. Review. - β-amyloid and tau drive early Alzheimer's disease decline while glucose hypometabolism drives late decline.
Hammond TC, Xing X, Wang C, Ma D, Nho K, Crane PK, Elahi F, Ziegler DA, Liang G, Cheng Q, Yanckello LM, Jacobs N, Lin AL. Hammond TC, et al. Commun Biol. 2020 Jul 6;3(1):352. doi: 10.1038/s42003-020-1079-x. Commun Biol. 2020. PMID: 32632135 Free PMC article. - Association of Methyl Donor Nutrients' Dietary Intake and Cognitive Impairment in the Elderly Based on the Intestinal Microbiome.
Chen Q, Fan R, Song L, Wang S, You M, Cai M, Wu Y, Li Y, Xu M. Chen Q, et al. Nutrients. 2024 Jun 28;16(13):2061. doi: 10.3390/nu16132061. Nutrients. 2024. PMID: 38999809 Free PMC article. - Interorgan communication with the liver: novel mechanisms and therapeutic targets.
Zhao J, Zhang X, Li Y, Yu J, Chen Z, Niu Y, Ran S, Wang S, Ye W, Luo Z, Li X, Hao Y, Zong J, Xia C, Xia J, Wu J. Zhao J, et al. Front Immunol. 2023 Dec 12;14:1314123. doi: 10.3389/fimmu.2023.1314123. eCollection 2023. Front Immunol. 2023. PMID: 38155961 Free PMC article. Review. - Longitudinal Analysis of the Microbiome and Metabolome in the 5xfAD Mouse Model of Alzheimer's Disease.
Dunham SJB, McNair KA, Adams ED, Avelar-Barragan J, Forner S, Mapstone M, Whiteson KL. Dunham SJB, et al. mBio. 2022 Dec 20;13(6):e0179422. doi: 10.1128/mbio.01794-22. Epub 2022 Dec 5. mBio. 2022. PMID: 36468884 Free PMC article.
References
- Alzheimer's A. 2017 Alzheimer's disease facts and figures. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2017;13:325–73.
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG019771/AG/NIA NIH HHS/United States
- R00 LM011384/LM/NLM NIH HHS/United States
- K99 LM011384/LM/NLM NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- R01 LM011360/LM/NLM NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- RF1 AG057452/AG/NIA NIH HHS/United States
- R01 AG048015/AG/NIA NIH HHS/United States
- RF1 AG051550/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- R01 AG046171/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- R03 AG054936/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- R01 MH098260/MH/NIMH NIH HHS/United States
- R01 EB022574/EB/NIBIB NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- K01 AG049050/AG/NIA NIH HHS/United States
- R01 LM012535/LM/NLM NIH HHS/United States
- U01 AG061359/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical