Magnetic resonance imaging in patients with cardiac implantable electronic devices - PubMed (original) (raw)
Review
Magnetic resonance imaging in patients with cardiac implantable electronic devices
A H Maass et al. Neth Heart J. 2018 Dec.
Abstract
In recent years the prevalence of implantation of a cardiac implantable electronic device (CIED) has increased due to expanding implantation indications and prolonged life expectancy. Diagnostic strategies increasingly employ magnetic resonance imaging (MRI) to aid therapeutic strategies. In earlier guidelines, MRI was contra-indicated in patients with CIEDs, mainly due to previous reports of severe complications. With the development of MRI-conditional CIEDs and recent evidence concerning non-MRI-conditional CIEDs, MRIs in CIED patients can be safely performed in many hospitals.However, there are several questions that need to be addressed. Which patients can we scan? How can the scans be performed safely? And last but not least, can cardiac MRI provide diagnostic yield in patients with CIEDs?Current European guidelines are rather outdated and vague about patient selection and practical issues. There are national guidelines on this topic but several issues need extra attention and those are addressed in this point of view. It is important to create an environment with proper patient selection without unnecessary MRI scans in CIED patients, but also without unnecessary fear of complications, preventing access to MRI in patients who can benefit from this powerful diagnostic tool.
Keywords: ICD; MRI; Pacemaker; Safety.
Conflict of interest statement
A.H. Maass received lecture fees from Medtronic and LivaNova. M.E.W. Hemels received lecture fees from Biotronik and LivaNova. C.P. Allaart received research grants from Boston Scientific and Abbott and lecture fees from Biotronik.
Figures
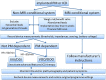
Fig. 1
Flow diagram of patient selection and device programming for magnetic resonance imaging. MRI magnetic resonance imaging, HR heart rate, PM pacemaker, ICD implantable cardioverter defibrillator, CS coronary sinus, ECG electrocardiogram
Similar articles
- State-of-the-art approach towards magnetic resonance imaging of the nervous system structures in patients with cardiac implantable electronic devices.
Gniadek-Olejniczak K, Makowski K, Olszewski A, Tomczykiewicz K, Krawczyk A, Mróz J. Gniadek-Olejniczak K, et al. Neurol Neurochir Pol. 2018 Nov-Dec;52(6):652-656. doi: 10.1016/j.pjnns.2018.07.002. Epub 2018 Jul 20. Neurol Neurochir Pol. 2018. PMID: 30061002 Review. - Provision of MR imaging for patients with cardiac implantable electronic devices (CIEDs): a single-center experience and national survey.
Murray AS, Gilligan PJ, Bisset JM, Nolan C, Galvin JM, Murray JG. Murray AS, et al. Ir J Med Sci. 2019 Aug;188(3):999-1004. doi: 10.1007/s11845-018-1922-y. Epub 2018 Oct 27. Ir J Med Sci. 2019. PMID: 30368645 - Remote programming of cardiac implantable electronic devices: A novel approach to program cardiac devices for magnetic resonance imaging.
Siddamsetti S, Shinn A, Gautam S. Siddamsetti S, et al. J Cardiovasc Electrophysiol. 2022 May;33(5):1005-1009. doi: 10.1111/jce.15434. Epub 2022 Mar 12. J Cardiovasc Electrophysiol. 2022. PMID: 35243710 - Access to MRI in Patients With Cardiac Implantable Electronic Devices is Variable and an Issue in Australia.
Page N, Chia K, Brazier D, Manisty C, Kozor R. Page N, et al. Heart Lung Circ. 2024 Mar;33(3):362-367. doi: 10.1016/j.hlc.2023.11.020. Epub 2024 Feb 7. Heart Lung Circ. 2024. PMID: 38326134 - Central nervous system MRI and cardiac implantable electronic devices.
Cadieu R, Peron M, Le Ven F, Kerdraon S, Boutet C, Mansourati J, Ben Salem D. Cadieu R, et al. J Neuroradiol. 2017 Feb;44(1):1-9. doi: 10.1016/j.neurad.2016.09.004. Epub 2016 Nov 1. J Neuroradiol. 2017. PMID: 27814887 Review.
Cited by
- Role of Cardiac MRI Imaging of Focal and Diffuse Inflammation and Fibrosis in Cardiomyopathy Patients Who Have Pacemakers/ICD Devices.
Zaman A, Zhao S, Kron J, Abbate A, Tomdio A, Hundley WG, Jordan JH. Zaman A, et al. Curr Cardiol Rep. 2022 Nov;24(11):1529-1536. doi: 10.1007/s11886-022-01770-w. Epub 2022 Aug 19. Curr Cardiol Rep. 2022. PMID: 35984554 Free PMC article. Review. - Is diversity harmful?-Mixed-brand cardiac implantable electronic devices undergoing magnetic resonance imaging.
König CA, Tinhofer F, Puntus T, Burger AL, Neubauer N, Langenberger H, Huber K, Nürnberg M, Zweiker D. König CA, et al. Wien Klin Wochenschr. 2022 Apr;134(7-8):286-293. doi: 10.1007/s00508-021-01924-w. Epub 2021 Aug 17. Wien Klin Wochenschr. 2022. PMID: 34402991 Free PMC article. - Cardiovascular magnetic resonance images with susceptibility artifacts: artificial intelligence with spatial-attention for ventricular volumes and mass assessment.
Penso M, Babbaro M, Moccia S, Guglielmo M, Carerj ML, Giacari CM, Chiesa M, Maragna R, Rabbat MG, Barison A, Martini N, Pepi M, Caiani EG, Pontone G. Penso M, et al. J Cardiovasc Magn Reson. 2022 Nov 28;24(1):62. doi: 10.1186/s12968-022-00899-5. J Cardiovasc Magn Reson. 2022. PMID: 36437452 Free PMC article. - Evolving use and indications for implantable cardioverter defibrillators.
de Groot JR. de Groot JR. Neth Heart J. 2018 Dec;26(12):581-583. doi: 10.1007/s12471-018-1193-2. Neth Heart J. 2018. PMID: 30426329 Free PMC article. No abstract available. - Safety of Magnetic Resonance Imaging in Patients with Cardiac Implantable Electronic Devices.
Lanz H, Strauß K, Höpler J, Kraft M, Hoffmann S, Binzenhöfer L, Gade N, Roden D, Saleh I, Kääb S, Lackermair K, Sadoni S, Hagl C, Massberg S, Estner H, Fichtner S, Lüsebrink E. Lanz H, et al. J Cardiovasc Dev Dis. 2024 Oct 8;11(10):313. doi: 10.3390/jcdd11100313. J Cardiovasc Dev Dis. 2024. PMID: 39452284 Free PMC article.
References
- van Veldhuisen DJ, Maass AH, Priori SG, et al. Implementation of device therapy (cardiac resynchronization therapy and implantable cardioverter defibrillator) for patients with heart failure in Europe: changes from 2004 to 2008. Eur J Heart Fail. 2009;11(12):1143–1151. doi: 10.1093/eurjhf/hfp149. - DOI - PubMed
- Williamson BD, Gohn DC, Ramza BM, et al. Real-World Evaluation of Magnetic Resonance Imaging in Patients With a Magnetic Resonance Imaging Conditional Pacemaker System: Results of 4‑Year Prospective Follow-Up in 2,629 Patients. Jacc Clin Electrophysiol. 2017;3(11):1231–1239. doi: 10.1016/j.jacep.2017.05.011. - DOI - PubMed
- European Society of C. European Heart Rhythm A. Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Europace. 2013;15(8):1070–1118. doi: 10.1093/europace/eut206. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources