Carvacrol reduces adipogenic differentiation by modulating autophagy and ChREBP expression - PubMed (original) (raw)
Carvacrol reduces adipogenic differentiation by modulating autophagy and ChREBP expression
Sonia Spalletta et al. PLoS One. 2018.
Abstract
Objective: Obesity is the result of white adipose tissue accumulation where excess of food energy is stored to form triglycerides. De novo lipogenesis (DNL) is the continuous process of new fat production and is driven by the transcription factor ChREBP. During adipogenesis, white adipocytes change their morphology and the entire cell volume is occupied by one large lipid droplet. Recent studies have implicated an essential role of autophagy in adipogenic differentiation, cytoplasmic remodelling and mitochondria reorganization. The phenolic monoterpenoid carvacrol (2-methyl-5-[1-methylethyl]phenol), produced by numerous aromatic plants, has been shown to reduce lipid accumulation in murine 3T3-L1 cells during adipogenic differentiation by modulating genes associated with adipogenesis and inflammation. Therefore, the aim of this study was to evaluate whether carvacrol could affect autophagy and ChREBP expression during adipogenic differentiation.
Methods: The study was carried on by using the murine 3T3-L1 and the human WJ-MSCs (Wharton's jelly-derived mesenchymal stem cells) cell lines. Cells undergoing adipogenic differentiation were untreated or treated with carvacrol. Adipogenic differentiation was assessed by analyzing cellular lipid accumulation with Oil-Red O staining and by ultrastructural examination with TEM. Autophagy was evaluated by western immunoblotting of autophagy markers LC3B and p62/SQSTM and by ultrastructural examination of autophagic bodies. Autophagic flux was evaluated by using autophagy inhibitor cloroquine (CQ). ChREBP expression levels was assessed by both western blotting and immunoelectron microscopy and ChREBP activity by analysis of adipogenic target genes expression.
Results: We found that carvacrol reduced adipogenic differentiation of about 40% and 30% in, respectively, 3T3-L1 and in WJ-MSCs cells. The effect of carvacrol on adipogenic differentiation correlated with both reduction of autophagy and reduction of ChREBP expression.
Conclusion: The results support the notion that carvacrol, through its effect on autophagy (essential for adipocyte maturation) and on ChREBP activity, could be used as a valuable adjuvant to reduce adipogenic differentiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Fig 1. Chemical structure of carvacrol.
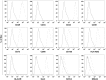
Fig 2. Cytofluorimetric analysis of the WJ cell culture.
The histograms show the cytofluorimetric analysis of the WJ cell culture, surface and intracellular antigens expression profile: CD29, CD34, CD44, CD45, CD73, CD90, CD105, OCT3/4, SSEA4 and Sox2. Gray histograms represent cells stained with the expression markers; black histograms are the respective IgG isotype control.
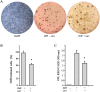
Fig 3. Oil Red Oil O staining.
3T3-L1 preadipocytes were differentiated in D-MEM (1-methyl 3-isobutylxanthine, dexamethasone, and insulin) medium for 7 days. Triglyceride accumulation visualized by Oil-Red O staining. Mature adipocytes show red large lipid vacuoles that occupied most of the cytoplasm. Original magnification: 400x.
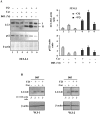
Fig 4. Reduction of adipocyte differentiation by carvacrol in 3T3-L1 cells.
3T3-L1 preadipocytes were grown in differentiation medium for 7 days (totally 90% differentiation) and treated with 25μ M carvacrol. After 7 days of adipogenic differentiation, visualization of triglyceride accumulation by Oil-Red O staining was conducted. (A) Cells were observed at optical Zeiss microscope and photographed. (B) Four adjacent 1 mm squares were counted, blind to group, using an inverted microscope and using criterion described for quantification of adipocyte differentiation [48]. The percentage of cells that underwent adipogenic differentiation was expressed as number of cells Red Oil positive/total. (C) Lipid accumulation was measured through a spectrophotometer. Data are presented as mean ± SD (n 3). * p<0.05 (differentiated + carvacrol vs differentiated cells).
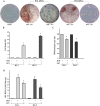
Fig 5. Reduction of adipocyte differentiation by carvacrol in WJ-MSCs cells.
Two subcultures of Wharton’s jelly (WJ-MSCs), WJ-1 and WJ-2 cells, were grown in differentiation medium for 17 days (totally 80% differentiation) and treated with 25 μM carvacrol. (A) Triglyceride accumulation was visualized by Oil-Red O staining. Cells were observed at optical Zeiss microscope and photographed. (B) After 17 days of differentiation, cell viability was measured by Trypan blue staining; graph shows percentage of detached dead cells. (C) Four adjacent 1 mm squares were counted, blind to group, using an inverted microscope. The percentage of cells that underwent adipogenic differentiation was expressed as number of Oil-Red O positive/total cells. (D) Lipid accumulation was measured by using spectrophotometer. Data are presented as mean ± SD (n 3). * p<0.05 (differentiated + carvacrol vs differentiated cells).
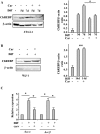
Fig 6. Effect of carvacrol on autophagy during adipogenic differentiation.
Western blot and densitometric analysis of LC3 and p62 expression in 3T3-L1 (A) and WJ-1 and WJ-2 (B) cells undergoing adipogenic medium for 7 days (3T3-L1) and 17 days (WJ), respectively, with or without carvacrol treatment. Densitometric analysis of the 3T3-L1 signals is shown in the right panel. Bars depict means ± SE. (n 2). * p<0.05. Lysosomal inhibitor Cloroquine (CQ) was added at 25μm for 4h before lysing cells for western blot analysis. Anti β-actin was used as protein loading control.
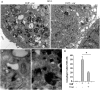
Fig 7. Transmission electron microscopy features of mature adipocyte.
(A) Human WJ-1 cells cultured for 17 days in adipogenic differentiation medium without or with 25 μM carvacrol. In the left panel is shown the adipogenic differentiation with visible lipid vacuoles (L); in the right panel is shown the inhibition of adipogenic differentiation after carvacrol co-treatment with reduction of lipid vacuoles. The arrows indicate the autophagy bodies formed during adipogenic differentiation. Note that the formation of autophagy bodies was considerably reduced by carvacrol treatment. (B) (C) High magnification of autophagy bodies in Diff-carv sample. (D) Autophagic bodies were counted on 11000 magnification image and expressed as total x 100 cells. Bars depict means ± SD. (n 3). * p<0.05. Nu:nucleus, cy:cytoplasm, L: lipid vacuoles. RER: rough endoplasmic reticulum. Original magnification: (A), x5600; (B) (C) x44000.
Fig 8. Effect of carvacrol on ChREBP acrivity during adipogenic differentiation.
Western blot and densitometric analysis of ChREBP in 3T3-L1 (A) and WJ-1 (B) cells undergoing adipogenic differentiation for, respectively, 5 to 7 days and 17 days with or without carvacrol co-treatment. Anti β-actin was used as protein loading control. (C) Expression of ChREBP target genes encoding enzymes involved in lipogenesis in WJ-1 cells treated as in (B). ChREBP target genes were detected by RT-PCR analysis and gene expression, measured by densitometry, was normalized to 28S levels, ±SD and plotted as relative mRNA expression. *p< 0.05 (diff compared to undiff; and diff+carv compared to diff). nss = not statistically significant.
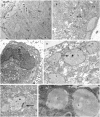
Fig 9. Immunogold detection of ChREBP protein in human WJ-MSCs cells.
Electron micrographs of uncounterstained sections of human WJ-1 cells cultured for 17 days in adipogenic differentiation medium with or without 25 μM carvacrol. (A) Undifferentiated: 20nm gold particles are distributed in nuclear and cytoplasmic compartments. (B) Differentiated cells in presence of carvacrol: cells are lower differentiated, 20-nmgold granules are distributed in nuclear and cytoplasmic compartments, inside the rough endoplasmic reticulum (RER). (C) Differentiated cells without carvacrol (control). (D) (E) (F) higher magnification of (C). The gold particles detected showed a predominant localization in the rough endoplasmic reticulum (C) mitochondria (E), lipid vacuoles (D) (F). Squares (10 cm2 at x 11000) were randomly chosen to count the number of 20-nm gold particles in the various cell compartments. Nu: nucleus, cy: cytoplasm, L: lipid vacuoles. RER: rough endoplasmic reticulum. M: mitochondrion. Arrows indicate gold clusters Original magnification: (A),(B),(C) x11000; (D),(E),(F) x22000.
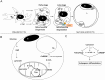
Fig 10. Schematic model showing the hypothesis of how carvacrol reduced adipogenesis.
Schematic representation showing the (A) formation of the mature adipocyte during adipogenic differentiation and the role of autophagy; (B) the role of glucose and ChREBP during TG formation; and (C) how carvacrol might reduce adipogenic differentiation. N: nucleus; A:autophagosome; L:lipid droplet; M:mitochondrion.
Similar articles
- Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity.
Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann JM, Nigro C, Mirra P, Fiory F, Formisano P, Miele C, Smith U, Beguinot F. Longo M, et al. Diabetologia. 2018 Feb;61(2):369-380. doi: 10.1007/s00125-017-4471-4. Epub 2017 Oct 24. Diabetologia. 2018. PMID: 29067487 Free PMC article. - Carvacrol prevents diet-induced obesity by modulating gene expressions involved in adipogenesis and inflammation in mice fed with high-fat diet.
Cho S, Choi Y, Park S, Park T. Cho S, et al. J Nutr Biochem. 2012 Feb;23(2):192-201. doi: 10.1016/j.jnutbio.2010.11.016. Epub 2011 Mar 29. J Nutr Biochem. 2012. PMID: 21447440 - The Glucose Sensor ChREBP Links De Novo Lipogenesis to PPARγ Activity and Adipocyte Differentiation.
Witte N, Muenzner M, Rietscher J, Knauer M, Heidenreich S, Nuotio-Antar AM, Graef FA, Fedders R, Tolkachov A, Goehring I, Schupp M. Witte N, et al. Endocrinology. 2015 Nov;156(11):4008-19. doi: 10.1210/EN.2015-1209. Epub 2015 Jul 16. Endocrinology. 2015. PMID: 26181104 - The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms.
da Silva C, Durandt C, Kallmeyer K, Ambele MA, Pepper MS. da Silva C, et al. Int J Mol Sci. 2020 Jun 9;21(11):4104. doi: 10.3390/ijms21114104. Int J Mol Sci. 2020. PMID: 32526833 Free PMC article. Review. - A cellular perspective of adipogenesis transcriptional regulation.
Kuri-Harcuch W, Velez-delValle C, Vazquez-Sandoval A, Hernández-Mosqueira C, Fernandez-Sanchez V. Kuri-Harcuch W, et al. J Cell Physiol. 2019 Feb;234(2):1111-1129. doi: 10.1002/jcp.27060. Epub 2018 Aug 26. J Cell Physiol. 2019. PMID: 30146705 Review.
Cited by
- Therapeutic application of carvacrol: A comprehensive review.
Imran M, Aslam M, Alsagaby SA, Saeed F, Ahmad I, Afzaal M, Arshad MU, Abdelgawad MA, El-Ghorab AH, Khames A, Shariati MA, Ahmad A, Hussain M, Imran A, Islam S. Imran M, et al. Food Sci Nutr. 2022 Aug 3;10(11):3544-3561. doi: 10.1002/fsn3.2994. eCollection 2022 Nov. Food Sci Nutr. 2022. PMID: 36348778 Free PMC article. Review. - The Epithelial-to-Mesenchymal Transition as a Possible Therapeutic Target in Fibrotic Disorders.
Di Gregorio J, Robuffo I, Spalletta S, Giambuzzi G, De Iuliis V, Toniato E, Martinotti S, Conti P, Flati V. Di Gregorio J, et al. Front Cell Dev Biol. 2020 Dec 21;8:607483. doi: 10.3389/fcell.2020.607483. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33409282 Free PMC article. Review. - Targeting Autophagy as a Strategy for Developing New Vaccines and Host-Directed Therapeutics Against Mycobacteria.
Strong EJ, Lee S. Strong EJ, et al. Front Microbiol. 2021 Jan 14;11:614313. doi: 10.3389/fmicb.2020.614313. eCollection 2020. Front Microbiol. 2021. PMID: 33519771 Free PMC article. Review. - Nigella Plants - Traditional Uses, Bioactive Phytoconstituents, Preclinical and Clinical Studies.
Salehi B, Quispe C, Imran M, Ul-Haq I, Živković J, Abu-Reidah IM, Sen S, Taheri Y, Acharya K, Azadi H, Del Mar Contreras M, Segura-Carretero A, Mnayer D, Sethi G, Martorell M, Abdull Razis AF, Sunusi U, Kamal RM, Rasul Suleria HA, Sharifi-Rad J. Salehi B, et al. Front Pharmacol. 2021 Apr 26;12:625386. doi: 10.3389/fphar.2021.625386. eCollection 2021. Front Pharmacol. 2021. PMID: 33981219 Free PMC article. Review. - MicroRNA-140-3p alleviates intervertebral disc degeneration via KLF5/N-cadherin/MDM2/Slug axis.
Wang Z, Zhang S, Zhao Y, Qu Z, Zhuang X, Song Q, Leng J, Liu Y. Wang Z, et al. RNA Biol. 2021 Dec;18(12):2247-2260. doi: 10.1080/15476286.2021.1898176. Epub 2021 Apr 27. RNA Biol. 2021. PMID: 33904383 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical