Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study - PubMed (original) (raw)
Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study
Peter Ueda et al. BMJ. 2018.
Abstract
Objective: To assess the association between the use of sodium glucose cotransporter 2 (SGLT2) inhibitors and seven serious adverse events of current concern.
Design: Register based cohort study.
Setting: Sweden and Denmark from July 2013 to December 2016.
Participants: A propensity score matched cohort of 17 213 new users of SGLT2 inhibitors (dapagliflozin, 61%; empagliflozin, 38%; canagliflozin, 1%) and 17 213 new users of the active comparator, glucagon-like peptide 1 (GLP1) receptor agonists.
Main outcome measures: The primary outcomes were lower limb amputation, bone fracture, diabetic ketoacidosis, acute kidney injury, serious urinary tract infection, venous thromboembolism, and acute pancreatitis, as identified from hospital records. Hazard ratios and 95% confidence intervals were estimated by using Cox proportional hazards models.
Results: Use of SGLT2 inhibitors, as compared with GLP1 receptor agonists, was associated with an increased risk of lower limb amputation (incidence rate 2.7 v 1.1 events per 1000 person years, hazard ratio 2.32, 95% confidence interval 1.37 to 3.91) and diabetic ketoacidosis (1.3 v 0.6, 2.14, 1.01 to 4.52) but not with bone fracture (15.4 v 13.9, 1.11, 0.93 to 1.33), acute kidney injury (2.3 v 3.2, 0.69, 0.45 to 1.05), serious urinary tract infection (5.4 v 6.0, 0.89, 0.67 to 1.19), venous thromboembolism (4.2 v 4.1, 0.99, 0.71 to 1.38) or acute pancreatitis (1.3 v 1.2, 1.16, 0.64 to 2.12).
Conclusions: In this analysis of nationwide registers from two countries, use of SGLT2 inhibitors, as compared with GLP1 receptor agonists, was associated with an increased risk of lower limb amputation and diabetic ketoacidosis, but not with other serious adverse events of current concern.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and have the following declarations: CJ reports personal fees from Pfizer and Bayer outside the submitted work; BE reports personal fees from Amgen, AstraZeneca, Boerhringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Mundipharma, Navamedic, Novo Nordisk, and RLS Global outside the submitted work, and grants from Sanofi outside the submitted work; and SG reports personal fees and research grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Novo Nordisk, and Sanofi outside of the submitted work. The other authors did not have any potential conflicts to report.
Figures
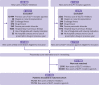
Fig 1
Flowchart of patient inclusion in the study cohort. SGLT2=sodium glucose cotransporter 2; GLP1=glucagon-like peptide 1. *A patient could be excluded for more than one reason.
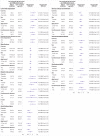
Fig 2
Subgroup analyses of serious adverse events among sodium glucose cotransporter 2 (SGLT2) inhibitor users compared with glucagon-like peptide 1 (GLP1) receptor agonist users. P values for homogeneity (all >0.05) are shown in supplementary materials, table 13. *Number of events not specified for <3 according to regulations on reporting Danish register data.
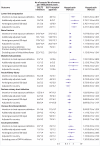
Fig 3
Sensitivity analyses of serious adverse events among sodium glucose cotransporter 2 (SGLT2) inhibitor users compared with glucagon-like peptide 1 (GLP1) receptor agonist users. DPP4=dipeptidyl peptidase 4.
Comment in
- Hydration must be maintained in patients taking sodium glucose cotransporter 2 inhibitors.
Auersperg EV. Auersperg EV. BMJ. 2019 Feb 6;364:l547. doi: 10.1136/bmj.l547. BMJ. 2019. PMID: 30728150 No abstract available.
Similar articles
- Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study.
Pasternak B, Wintzell V, Melbye M, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Svanström H, Ueda P. Pasternak B, et al. BMJ. 2020 Apr 29;369:m1186. doi: 10.1136/bmj.m1186. BMJ. 2020. PMID: 32349963 Free PMC article. - SGLT2 inhibitors for diabetes are linked to increased risk of lower limb amputation.
Kmietowicz Z. Kmietowicz Z. BMJ. 2018 Nov 14;363:k4828. doi: 10.1136/bmj.k4828. BMJ. 2018. PMID: 30429136 No abstract available. - Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials.
Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Jabbour S, et al. Diabetes Obes Metab. 2018 Mar;20(3):620-628. doi: 10.1111/dom.13124. Epub 2017 Oct 26. Diabetes Obes Metab. 2018. PMID: 28950419 Free PMC article. Review. - Impact of Sodium-Glucose Cotransporter 2 Inhibitors on Nonglycemic Outcomes in Patients with Type 2 Diabetes.
Trujillo JM, Nuffer WA. Trujillo JM, et al. Pharmacotherapy. 2017 Apr;37(4):481-491. doi: 10.1002/phar.1903. Epub 2017 Feb 24. Pharmacotherapy. 2017. PMID: 28102030 Free PMC article. Review.
Cited by
- Trends in Antidiabetic Drug Use and Safety of Metformin in Diabetic Patients with Varying Degrees of Chronic Kidney Disease from 2010 to 2021 in Korea: Retrospective Cohort Study Using the Common Data Model.
Joo SH, Yang S, Lee S, Park SJ, Park T, Rhee SY, Cha JM, Rhie SJ, Hwang HS, Kim YG, Chung EK. Joo SH, et al. Pharmaceuticals (Basel). 2024 Oct 14;17(10):1369. doi: 10.3390/ph17101369. Pharmaceuticals (Basel). 2024. PMID: 39459008 Free PMC article. - Differences in the Adverse Event Profiles of Sodium-Glucose Cotransporter 2 Inhibitors used in Patients with Diabetes Mellitus and Heart Failure: An Analysis Using the Japanese Adverse Drug Event Report Database.
Sakamoto T, Miyamoto H, Hashizume J, Akamatsu H, Akagi T, Kodama Y, Hamano H, Zamami Y, Ohyama K. Sakamoto T, et al. Clin Drug Investig. 2024 Oct;44(10):761-771. doi: 10.1007/s40261-024-01394-8. Epub 2024 Oct 14. Clin Drug Investig. 2024. PMID: 39402407 - Cardiovascular Effectiveness and Safety of Antidiabetic Drugs in Patients with Type 2 Diabetes and Peripheral Artery Disease: Systematic Review.
Cimellaro A, Cavallo M, Mungo M, Suraci E, Spagnolo F, Addesi D, Pintaudi M, Pintaudi C. Cimellaro A, et al. Medicina (Kaunas). 2024 Sep 20;60(9):1542. doi: 10.3390/medicina60091542. Medicina (Kaunas). 2024. PMID: 39336583 Free PMC article. Review. - Benefit and Safety of Sodium-Glucose Co-Transporter 2 Inhibitors in Older Patients with Type 2 Diabetes Mellitus.
Jeon JY, Kim DJ. Jeon JY, et al. Diabetes Metab J. 2024 Sep;48(5):837-846. doi: 10.4093/dmj.2024.0317. Epub 2024 Sep 12. Diabetes Metab J. 2024. PMID: 39313229 Free PMC article. Review.
References
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM475487.pdf.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). https://www.fda.gov/downloads/Drugs/DrugSafety/UCM506772.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical