Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial - PubMed (original) (raw)
Randomized Controlled Trial
Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial
Cara B Ebbeling et al. BMJ. 2018.
Erratum in
- Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial.
[No authors listed] [No authors listed] BMJ. 2020 Nov 3;371:m4264. doi: 10.1136/bmj.m4264. BMJ. 2020. PMID: 33144344 Free PMC article. No abstract available.
Abstract
Objective: To determine the effects of diets varying in carbohydrate to fat ratio on total energy expenditure.
Design: Randomized trial.
Setting: Multicenter collaboration at US two sites, August 2014 to May 2017.
Participants: 164 adults aged 18-65 years with a body mass index of 25 or more.
Interventions: After 12% (within 2%) weight loss on a run-in diet, participants were randomly assigned to one of three test diets according to carbohydrate content (high, 60%, n=54; moderate, 40%, n=53; or low, 20%, n=57) for 20 weeks. Test diets were controlled for protein and were energy adjusted to maintain weight loss within 2 kg. To test for effect modification predicted by the carbohydrate-insulin model, the sample was divided into thirds of pre-weight loss insulin secretion (insulin concentration 30 minutes after oral glucose).
Main outcome measures: The primary outcome was total energy expenditure, measured with doubly labeled water, by intention-to-treat analysis. Per protocol analysis included participants who maintained target weight loss, potentially providing a more precise effect estimate. Secondary outcomes were resting energy expenditure, measures of physical activity, and levels of the metabolic hormones leptin and ghrelin.
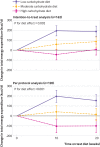
Results: Total energy expenditure differed by diet in the intention-to-treat analysis (n=162, P=0.002), with a linear trend of 52 kcal/d (95% confidence interval 23 to 82) for every 10% decrease in the contribution of carbohydrate to total energy intake (1 kcal=4.18 kJ=0.00418 MJ). Change in total energy expenditure was 91 kcal/d (95% confidence interval -29 to 210) greater in participants assigned to the moderate carbohydrate diet and 209 kcal/d (91 to 326) greater in those assigned to the low carbohydrate diet compared with the high carbohydrate diet. In the per protocol analysis (n=120, P<0.001), the respective differences were 131 kcal/d (-6 to 267) and 278 kcal/d (144 to 411). Among participants in the highest third of pre-weight loss insulin secretion, the difference between the low and high carbohydrate diet was 308 kcal/d in the intention-to-treat analysis and 478 kcal/d in the per protocol analysis (P<0.004). Ghrelin was significantly lower in participants assigned to the low carbohydrate diet compared with those assigned to the high carbohydrate diet (both analyses). Leptin was also significantly lower in participants assigned to the low carbohydrate diet (per protocol).
Conclusions: Consistent with the carbohydrate-insulin model, lowering dietary carbohydrate increased energy expenditure during weight loss maintenance. This metabolic effect may improve the success of obesity treatment, especially among those with high insulin secretion.
Trial registration: ClinicalTrials.gov NCT02068885.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf and declare: research support for the submitted work as described above; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work except as follows: CBE and DSL have conducted research studies examining the carbohydrate-insulin model funded by the National Institutes of Health and philanthropic organizations unaffiliated with the food industry; DSL received royalties for books on obesity and nutrition that recommend a low glycemic load diet.
Figures
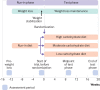
Fig 1
Study design
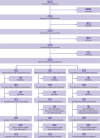
Fig 2
Participant flow (see supplementary figure for details of exclusions)
Fig 3
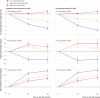
Change in total energy expenditure, the primary outcome, in intention-to-treat (top) and per protocol (bottom) analyses. Data are shown as mean change from start of test phase, with whiskers representing 1 standard error above and below the mean. P tests uniformity across diet groups for average of changes at midpoint and end of test phase
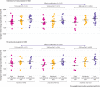
Fig 4
Effect modification by pre-weight loss insulin secretion (insulin concentration 30 minutes after oral glucose) in intention-to-treat and per protocol analyses. Pre-weight loss body weight differed by third (first third, 83.8 kg; second third, 92.8 kg; third third 98.4 kg, P<0.001 in the intention-to-treat analysis). Change in body weight during the test phase did not differ by third (P=0.08) or across diet groups (P=0.43)
Fig 5
Biomeasures of compliance in intention-to-treat and per protocol analyses. Measures include 1,5-anhydroglucitol (upper), mean pre-weight loss value 17 μg/mL; triglycerides (middle), mean pre-weight loss value 78 mg/dL (retransformed); and high density lipoprotein cholesterol (lower), mean pre-weight loss value 48 mg/dL. Data are shown as mean change from start of test phase, with whiskers representing 1 standard error above and below the mean. P tests uniformity across diet groups for average of changes at midpoint and end of test phase. Left, intention-to-treat analysis; right, per-protocol analysis
Comment in
- During weight-loss maintenance, energy expenditure was higher with lower-carbohydrate diets.
Kahan S. Kahan S. Ann Intern Med. 2019 Mar 19;170(6):JC31. doi: 10.7326/ACPJ201903190-031. Ann Intern Med. 2019. PMID: 30884501 No abstract available.
Similar articles
- Effects of dietary composition on energy expenditure during weight-loss maintenance.
Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Ebbeling CB, et al. JAMA. 2012 Jun 27;307(24):2627-34. doi: 10.1001/jama.2012.6607. JAMA. 2012. PMID: 22735432 Free PMC article. Clinical Trial. - Energy Requirement Is Higher During Weight-Loss Maintenance in Adults Consuming a Low- Compared with High-Carbohydrate Diet.
Ebbeling CB, Bielak L, Lakin PR, Klein GL, Wong JMW, Luoto PK, Wong WW, Ludwig DS. Ebbeling CB, et al. J Nutr. 2020 Aug 1;150(8):2009-2015. doi: 10.1093/jn/nxaa150. J Nutr. 2020. PMID: 32470981 Free PMC article. - A randomized study of dietary composition during weight-loss maintenance: Rationale, study design, intervention, and assessment.
Ebbeling CB, Klein GL, Luoto PK, Wong JMW, Bielak L, Eddy RG, Steltz SK, Devlin C, Sandman M, Hron B, Shimy K, Heymsfield SB, Wolfe RR, Wong WW, Feldman HA, Ludwig DS. Ebbeling CB, et al. Contemp Clin Trials. 2018 Feb;65:76-86. doi: 10.1016/j.cct.2017.12.004. Epub 2017 Dec 9. Contemp Clin Trials. 2018. PMID: 29233719 Free PMC article. - Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials.
Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Wycherley TP, et al. Am J Clin Nutr. 2012 Dec;96(6):1281-98. doi: 10.3945/ajcn.112.044321. Epub 2012 Oct 24. Am J Clin Nutr. 2012. PMID: 23097268 Review. - The effects of dietary macronutrient composition on resting energy expenditure following active weight loss: A systematic review and meta-analysis.
Ho DKN, Liao YC, Mayasari NR, Chien MM, Chung M, Bai CH, Huang YL, Chen YC, Tseng SH, Chang CC, Chiu WC, Sangopas P, Tseng HT, Kao JW, Ngu YJ, Chang JS. Ho DKN, et al. Obes Rev. 2024 Aug;25(8):e13760. doi: 10.1111/obr.13760. Epub 2024 May 2. Obes Rev. 2024. PMID: 38697953 Review.
Cited by
- Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice.
Minderis P, Fokin A, Povilonis T, Kvedaras M, Ratkevicius A. Minderis P, et al. Nutrients. 2023 Nov 19;15(22):4836. doi: 10.3390/nu15224836. Nutrients. 2023. PMID: 38004232 Free PMC article. - Dose-Dependent Associations of Dietary Glycemic Index, Glycemic Load, and Fiber With 3-Year Weight Loss Maintenance and Glycemic Status in a High-Risk Population: A Secondary Analysis of the Diabetes Prevention Study PREVIEW.
Zhu R, Larsen TM, Fogelholm M, Poppitt SD, Vestentoft PS, Silvestre MP, Jalo E, Navas-Carretero S, Huttunen-Lenz M, Taylor MA, Stratton G, Swindell N, Drummen M, Adam TC, Ritz C, Sundvall J, Valsta LM, Muirhead R, Brodie S, Handjieva-Darlenska T, Handjiev S, Martinez JA, Macdonald IA, Westerterp-Plantenga MS, Brand-Miller J, Raben A. Zhu R, et al. Diabetes Care. 2021 Jul;44(7):1672-1681. doi: 10.2337/dc20-3092. Epub 2021 May 27. Diabetes Care. 2021. PMID: 34045241 Free PMC article. Clinical Trial. - Advantages and Disadvantages of the Ketogenic Diet: A Review Article.
Batch JT, Lamsal SP, Adkins M, Sultan S, Ramirez MN. Batch JT, et al. Cureus. 2020 Aug 10;12(8):e9639. doi: 10.7759/cureus.9639. Cureus. 2020. PMID: 32923239 Free PMC article. Review. - Metabolic Characterization of Meat, Fish, and Soda Intake in Males: Secondary Results from a Randomized Inpatient Pilot Study.
Mitchell CM, Piaggi P, O'Brien DM, Krakoff J, Votruba SB. Mitchell CM, et al. Obesity (Silver Spring). 2021 Jun;29(6):995-1002. doi: 10.1002/oby.23167. Epub 2021 May 3. Obesity (Silver Spring). 2021. PMID: 33938613 Free PMC article. Clinical Trial. - Randomized controlled trial of a novel lifestyle intervention used with or without meal replacements in work sites.
Das SK, Silver RE, Vail TA, Chin MK, Blanchard CM, Dickinson SL, Chen X, Ceglia L, Saltzman E, Allison DB, Roberts SB. Das SK, et al. Obesity (Silver Spring). 2023 Feb;31(2):374-389. doi: 10.1002/oby.23636. Obesity (Silver Spring). 2023. PMID: 36695057 Free PMC article. Clinical Trial.