Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3 - PubMed (original) (raw)
Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3
In Jun Yeo et al. Arch Pharm Res. 2019 Mar.
Abstract
Ultraviolet B (UVB) irradiation causes sunburn, inflammatory responses, dysregulation of immune function, oxidative stress, DNA damage and photocarcinogenesis on skin. Rosemary (Rosmarinus officinalis L.) has been reported to inhibit inflammation. Carnosol, a major component of Rosemary, has prominent anti-inflammatory effects. However, its protective effect on UVB-induced inflammatory skin responses has not yet been reported. Here, we investigated the effectiveness of carnosol on UVB-induced inflammation. We examined the anti-inflammation effect of topical application of carnosol (0.05 µg/cm2) on UVB (540 mJ/cm2, for 3 successive days)-induced skin inflammation in HR1 mice. Topical application of carnosol inhibited UVB-induced erythema, epidermal thickness, inflammatory responses in HR1 mice. Carnosol reduced the level of Immunoglobulin-E and IL-1β in blood serum of UVB-induced mice. Carnosol also significantly inhibited the UVB-induced expression of inflammatory marker protein (iNOS and COX-2) in back skin of mice. In addition, carnosol treated skin decreased activation of STAT3, a transcriptional factor regulating inflammatory genes. Our study suggested that carnosol has protective effects on skin inflammatory skin damages by UVB.
Keywords: Carnosol; Dermatitis; STAT3; UVB.
Conflict of interest statement
All authors declare that there is no potential conflict of interest with respect to the authors and/or publications of this article.
Figures
Fig. 1
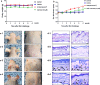
Differences in body weight, ear thickness, ear and back phenotypes and histology. UVB irradiation (540 mJ/cm2, 15 min a day for three successive days) was exposed during topical application of carnosol (0.05 µg/cm2). After 4 weeks, body weight (a) and ear thickness (b) were observed at least three times following the procedure described in Materials and Methods. *P < 0.05, significant difference compared to UVB treated group. Phenotypes (c) of mouse randomly selected from each group (1 mouse/group). Histopathology of ear and back skin in control (d-1), vehicle (d-2), UVB (d-3), and UVB + carnosol (d-4). Histopathological changes in the slide sections of ear and back tissue were identified by staining with hematoxylin and eosin followed by observation at × 200 magnification. Scale bars, 100 μm. Data shown are mean ± SD (n = 10)
Fig. 2
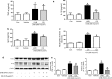
Changes in serum cytokine concentration, and expression of TNF-α, IL-1β in the back skin. After final treatment, mice from each group were sacrificed under anesthesia. Serum was used to measure cytokine concentration. It was prepared from blood sample collected from the abdominal vein of each mouse. Serum levels of TNF-α and IL-1β (a), IgE (b), were quantified by ELISA. Data shown are mean ± SD (n = 10). Blood cell (c, Neutrophile) number was measured by an automatic hematologic analyzer ADVIA2120 (Siemens Healthcare Diagnostics) in the laboratory animal research center of Chungbuk National University. *P < 0.05, significant difference compared to the control group. #P < 0.05, significant difference compared to UVB exposed group. Protein expression levels of TNF-α, and IL-1β in the back skin (d) were measured by Western blotting. Relative densities of protein bands were quantified (e) following the procedure described in Materials and methods. Equal amounts of total proteins (20 μg/lane) were subjected to 10% SDS-PAGE and expression levels of TNF-α and IL-1β protein were detected by Western blotting using specific antibodies. β-actin protein was used as an internal control. Data shown are mean ± SD (n = 2). *P < 0.05, significant difference compared to the control group. #P < 0.05, significantly different compared to UVB exposed group
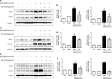
Fig. 3
Expression level of iNOS and COX-2 protein in lymph node and ear or back skin. Protein expression levels of iNOS and COX-2 in lymph node (a), ear skin (c), and back skin (e) were measured by Western blotting. Relative densities of protein bands were quantified (b, d, f) following the procedure described in Materials and methods. Equal amounts of total proteins (20 μg/lane) were subjected to 10% SDS-PAGE and expression levels of iNOS and COX-2 protein were detected by Western blotting using specific antibodies. β-actin protein was used as an internal control. Data shown are mean ± SD (n = 2). *P < 0.05, significant difference compared to the control group. #P < 0.05, significantly different compared to UVB exposed group
Fig. 4
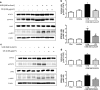
Expression level of STAT3 and p-STAT3 protein in ear or back skin. Protein expression levels of STAT3 and p-STAT3 in ear skin (a) and back skin (c) were measured by Western blotting. Relative densities of protein bands were quantified (b, d) following the procedure described in Materials and methods. Equal amounts of total proteins (20 μg/lane) were subjected to 10% SDS-PAGE and expression levels of STAT3 and p-STAT3 proteins were detected by Western blotting using specific antibodies. β-actin protein was used as an internal control. Data shown are mean ± SD (n = 2). *P < 0.05, significant difference compared to the control group. #P < 0.05, significantly different compared to UVB exposed group
Fig. 5
DNA binding actitiy of STAT3 in ear or back skin. DNA binding actitiy of STAT3 in ear skin (a) and back skin (z) were measured by EMSA, and densities of EMSA bands were quantified (b, d) following the procedure described in Materials and methods. Data shown are mean ± SD (n = 2). *P < 0.05, significant difference compared to the control group. #P < 0.05, significantly different compared to UVB exposed group
Similar articles
- Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3.
Lee DY, Hwang CJ, Choi JY, Park MH, Song MJ, Oh KW, Son DJ, Lee SH, Han SB, Hong JT. Lee DY, et al. Biomol Ther (Seoul). 2017 Sep 1;25(5):535-544. doi: 10.4062/biomolther.2017.006. Biomol Ther (Seoul). 2017. PMID: 28655070 Free PMC article. - The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation.
Tong L, Wu S. Tong L, et al. Sci Rep. 2018 Feb 23;8(1):3574. doi: 10.1038/s41598-018-22029-x. Sci Rep. 2018. PMID: 29476131 Free PMC article. - Hydroalcoholic extract of Rosemary (Rosmarinus officinalis L.) and its constituent carnosol inhibit formalin-induced pain and inflammation in mice.
Emami F, Ali-Beig H, Farahbakhsh S, Mojabi N, Rastegar-Moghadam B, Arbabian S, Kazemi M, Tekieh E, Golmanesh L, Ranjbaran M, Jalili C, Noroozzadeh A, Sahraei H. Emami F, et al. Pak J Biol Sci. 2013 Apr 1;16(7):309-16. doi: 10.3923/pjbs.2013.309.316. Pak J Biol Sci. 2013. PMID: 24498797 - The Dietary Components Carnosic Acid and Carnosol as Neuroprotective Agents: a Mechanistic View.
de Oliveira MR. de Oliveira MR. Mol Neurobiol. 2016 Nov;53(9):6155-6168. doi: 10.1007/s12035-015-9519-1. Epub 2015 Nov 9. Mol Neurobiol. 2016. PMID: 26553346 Review.
Cited by
- Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol.
Habtemariam S. Habtemariam S. Biomedicines. 2023 Feb 13;11(2):545. doi: 10.3390/biomedicines11020545. Biomedicines. 2023. PMID: 36831081 Free PMC article. Review. - Spice-Derived Phenolic Compounds: Potential for Skin Cancer Prevention and Therapy.
Baloghová J, Michalková R, Baranová Z, Mojžišová G, Fedáková Z, Mojžiš J. Baloghová J, et al. Molecules. 2023 Aug 25;28(17):6251. doi: 10.3390/molecules28176251. Molecules. 2023. PMID: 37687080 Free PMC article. Review. - Saireito Improves Lymphatic Function and Prevents UVB-Induced Acute Inflammation and Photodamage in HR-1 Hairless Mice.
Oyama M, Murata K, Ogata M, Fujita N, Takahashi R. Oyama M, et al. Evid Based Complement Alternat Med. 2021 Jun 25;2021:3707058. doi: 10.1155/2021/3707058. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34257677 Free PMC article. - RhFGF21 Protects Epidermal Cells against UVB-Induced Apoptosis through Activating AMPK-Mediated Autophagy.
Zhao Y, Lin J, Li J, Bwalya C, Xu Y, Niu Y, Zhang Y, Wu J, Xu Y, Chen J, Ye S, Lin L. Zhao Y, et al. Int J Mol Sci. 2022 Oct 18;23(20):12466. doi: 10.3390/ijms232012466. Int J Mol Sci. 2022. PMID: 36293323 Free PMC article. - Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases.
Li Pomi F, Papa V, Borgia F, Vaccaro M, Allegra A, Cicero N, Gangemi S. Li Pomi F, et al. Antioxidants (Basel). 2023 Mar 9;12(3):680. doi: 10.3390/antiox12030680. Antioxidants (Basel). 2023. PMID: 36978928 Free PMC article. Review.
References
- Amano W, Nakajima S, Kunugi H, Numata Y, Kitoh A, Egawa G, Dainichi T, Honda T, Otsuka A, Kimoto Y, Yamamoto Y, Tanimoto A, Matsushita M, Miyachi Y, Kabashima K. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol. 2015;136(667–677):e667. doi: 10.1016/j.jaci.2015.03.051. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous