Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: A multicenter open-label trial in Korea - PubMed (original) (raw)
Randomized Controlled Trial
. 2018 Nov;97(46):e13157.
doi: 10.1097/MD.0000000000013157.
Seong Heon Kim 2, Kyoung Hee Han 3, Hyun Jin Choi 4, Heeyeon Cho 5, Jung Won Lee 6, Jae Il Shin 7, Min Hyun Cho 8, Joo Hoon Lee 9, Young Seo Park 9, Il-Soo Ha 4, Hae Il Cheong 4, Su Young Kim 2, Seung Joo Lee 6, Hee Gyung Kang 4
Affiliations
- PMID: 30431588
- PMCID: PMC6257685
- DOI: 10.1097/MD.0000000000013157
Randomized Controlled Trial
Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: A multicenter open-label trial in Korea
Yo Han Ahn et al. Medicine (Baltimore). 2018 Nov.
Abstract
Background: The anti-CD20 monoclonal antibody rituximab (RTX) has been proposed as a rescue therapy for difficult-to-treat nephrotic syndrome (NS). We conducted a clinical trial to evaluate the efficacy and safety of RTX in children with difficult-to-treat NS dependent on or resistant to steroids and calcineurin inhibitors (CNIs).
Methods: A multicenter open-label trial was performed at 8 major pediatric nephrology centers in Korea. The investigation consisted of a randomized controlled trial for steroid- and CNI-dependent NS (DDNS; randomization into the RTX group and the control group, at a ratio of 2:1) and a single-arm study of steroid and CNI-resistant NS (DRNS). DDNS patients in the RTX group and DRNS patients received a single dose of intravenous RTX (375 mg/m of body surface area) for B-cell depletion. A second RTX dose was administered at week 2 if the first dose failed to achieve depletion of CD19(+) cells. The primary endpoint was rate of maintaining remission at 6 months after treatment for DDNS and rate of remission achievement for DRNS.
Results: Sixty-one children with DDNS were enrolled while in remission and randomized to the control group (21 patients) or the RTX group (40 patients). At 6 months after treatment, the remission rates were 74.3% in the RTX group and 31.3% in the control group (P = .003). The mean duration of remission maintenance was significantly higher in the RTX group than in the control group (9.0 vs 2.9 months, P = .004). Of the 23 patients with DRNS enrolled in the single-arm study and treated with RTX, 9 (39.1%) achieved partial or complete remission within 6 months. Depletion of B cells occurred in all patients with RTX therapy. Thirty patients (50.8% of 59 patients analyzed) experienced mild and transient infusion reaction during RTX administration, and most adverse events were mild.
Conclusions: RTX administration was safe and effective in patients with difficult-to-treat NS. One or 2 doses of RTX may be sufficient to deplete B cells and achieve better control of pediatric NS.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
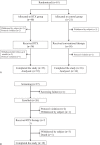
Figure 1
Study design. (A) Randomized controlled trial of drug-dependent nephrotic syndrome. (B) Single-arm study of drug-resistant nephrotic syndrome.
Figure 2
Flowchart of patient enrollment, evaluation, and follow-up. (A) Randomized controlled trial of drug-dependent nephrotic syndrome. (B) Single-arm study of drug-resistant nephrotic syndrome.
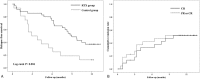
Figure 3
Survival analysis. (A) Kaplan–Meier analysis for relapse-free survival in the randomized controlled trial of drug-dependent nephrotic syndrome. (B) Cumulative remission rate in single-arm study of drug-resistant nephrotic syndrome.
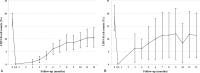
Figure 4
CD19 B-cell counts after rituximab therapy. (A) Randomized controlled trial of drug-dependent nephrotic syndrome. (B) Single-arm study of drug-resistant nephrotic syndrome.
Similar articles
- Human or Chimeric Monoclonal Anti-CD20 Antibodies for Children with Nephrotic Syndrome: A Superiority Randomized Trial.
Ravani P, Colucci M, Bruschi M, Vivarelli M, Cioni M, DiDonato A, Cravedi P, Lugani F, Antonini F, Prunotto M, Emma F, Angeletti A, Ghiggeri GM. Ravani P, et al. J Am Soc Nephrol. 2021 Oct;32(10):2652-2663. doi: 10.1681/ASN.2021040561. Epub 2021 Sep 20. J Am Soc Nephrol. 2021. PMID: 34544820 Free PMC article. Clinical Trial. - Cost analysis on the use of rituximab and calcineurin inhibitors in children and adolescents with steroid-dependent nephrotic syndrome.
Iorember F, Aviles D, Kallash M, Bamgbola O. Iorember F, et al. Pediatr Nephrol. 2018 Feb;33(2):261-267. doi: 10.1007/s00467-017-3789-y. Epub 2017 Sep 1. Pediatr Nephrol. 2018. PMID: 28864927 - Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: study protocol.
Ravani P, Bonanni A, Ghiggeri GM. Ravani P, et al. BMJ Open. 2017 Mar 17;7(3):e013319. doi: 10.1136/bmjopen-2016-013319. BMJ Open. 2017. PMID: 28314744 Free PMC article. Clinical Trial. - Rituximab in children with steroid-dependent nephrotic syndrome: experience of a tertiary center and review of the literature.
Van Horebeek I, Knops N, Van Dyck M, Levtchenko E, Mekahli D. Van Horebeek I, et al. Acta Clin Belg. 2017 Jun;72(3):147-155. doi: 10.1080/17843286.2016.1208955. Epub 2016 Jul 13. Acta Clin Belg. 2017. PMID: 27409338 Review. - Anti-CD20 Antibodies for Idiopathic Nephrotic Syndrome in Children.
Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM. Ravani P, et al. Clin J Am Soc Nephrol. 2016 Apr 7;11(4):710-20. doi: 10.2215/CJN.08500815. Epub 2015 Nov 19. Clin J Am Soc Nephrol. 2016. PMID: 26585985 Free PMC article. Review.
Cited by
- Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children.
Larkins NG, Hahn D, Liu ID, Willis NS, Craig JC, Hodson EM. Larkins NG, et al. Cochrane Database Syst Rev. 2024 Nov 8;11(11):CD002290. doi: 10.1002/14651858.CD002290.pub6. Cochrane Database Syst Rev. 2024. PMID: 39513526 Review. - Using real-world data to inform dosing strategies of rituximab for pediatric patients with frequent-relapsing or steroid-dependent nephrotic syndrome: a prospective pharmacokinetic-pharmacodynamic study.
Chen Y, Shen Q, Xiong Y, Dong M, Xu H, Li Z. Chen Y, et al. Front Pharmacol. 2024 Jan 9;14:1319744. doi: 10.3389/fphar.2023.1319744. eCollection 2023. Front Pharmacol. 2024. PMID: 38264525 Free PMC article. - Efficacy and safety of long-term repeated use of rituximab in pediatric patients with nephrotic syndrome.
Choi N, Min J, Kim JH, Kang HG, Ahn YH. Choi N, et al. Pediatr Nephrol. 2024 Mar;39(3):771-780. doi: 10.1007/s00467-023-06124-4. Epub 2023 Sep 8. Pediatr Nephrol. 2024. PMID: 37682369 - Long-Term Efficacy and Safety of Rituximab Versus Tacrolimus in Children With Steroid Dependent Nephrotic Syndrome.
Basu B, Erdmann S, Sander A, Mahapatra TKS, Meis J, Schaefer F. Basu B, et al. Kidney Int Rep. 2023 May 29;8(8):1575-1584. doi: 10.1016/j.ekir.2023.05.022. eCollection 2023 Aug. Kidney Int Rep. 2023. PMID: 37547526 Free PMC article. - Mycophenolic acid directly protects podocytes by preserving the actin cytoskeleton and increasing cell survival.
Abo Zed SED, Hackl A, Bohl K, Ebert L, Kieckhöfer E, Müller C, Becker K, Fink G, Nüsken KD, Nüsken E, Müller RU, Schermer B, Weber LT. Abo Zed SED, et al. Sci Rep. 2023 Mar 15;13(1):4281. doi: 10.1038/s41598-023-31326-z. Sci Rep. 2023. PMID: 36922538 Free PMC article.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:139–274.
- El-Husseini A, El-Basuony F, Mahmoud I, et al. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant 2005;20:2433–8. - PubMed
- Foster BJ, Shults J, Zemel BS, et al. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr 2004;80:1334–41. - PubMed
- Iijima K, Hamahira K, Tanaka R, et al. Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 2002;61:1801–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous