Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors - PubMed (original) (raw)
. 2018 Aug 26;3(5):575-587.
doi: 10.1016/j.jacbts.2018.07.006. eCollection 2018 Oct.
Sonia Rawat 2, Kim L Ho 2, Cory S Wagg 2, Liyan Zhang 2, Hwee Teoh 1 3, John E Dyck 2, Golam M Uddin 2, Gavin Y Oudit 2, Eric Mayoux 4, Michael Lehrke 5, Nikolaus Marx 5, Gary D Lopaschuk 2
Affiliations
- PMID: 30456329
- PMCID: PMC6234616
- DOI: 10.1016/j.jacbts.2018.07.006
Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors
Subodh Verma et al. JACC Basic Transl Sci. 2018.
Abstract
SGLT2 inhibitors have profound benefits on reducing heart failure and cardiovascular mortality in individuals with type 2 diabetes, although the mechanism(s) of this benefit remain poorly understood. Because changes in cardiac bioenergetics play a critical role in the pathophysiology of heart failure, the authors evaluated cardiac energy production and substrate use in diabetic mice treated with the SGTL2 inhibitor, empagliflozin. Empagliflozin treatment of diabetic db/db mice prevented the development of cardiac failure. Glycolysis, and the oxidation of glucose, fatty acids and ketones were measured in the isolated working heart perfused with 5 mmol/l glucose, 0.8 mmol/l palmitate, 0.5 mmol/l ß-hydroxybutyrate (ßOHB), and 500 μU/ml insulin. In vehicle-treated db/db mice, cardiac glucose oxidation rates were decreased by 61%, compared with control mice, but only by 43% in empagliflozin-treated diabetic mice. Interestingly, cardiac ketone oxidation rates in db/db mice decreased to 45% of the rates seen in control mice, whereas a similar decrease (43%) was seen in empagliflozin-treated db/db mice. Overall cardiac adenosine triphosphate (ATP) production rates decreased by 36% in db/db vehicle-treated hearts compared with control mice, with fatty acid oxidation providing 42%, glucose oxidation 26%, ketone oxidation 10%, and glycolysis 22% of ATP production in db/db mouse hearts. In empagliflozin-treated db/db mice, cardiac ATP production rates increased by 31% compared with db/db vehicle-treated mice, primarily due to a 61% increase in the contribution of glucose oxidation to energy production. Cardiac efficiency (cardiac work/O2 consumed) decreased by 28% in db/db vehicle-treated hearts, compared with control hearts, and empagliflozin did not increase cardiac efficiency per se. Because ketone oxidation was impaired in db/db mouse hearts, the authors determined whether this contributed to the decrease in cardiac efficiency seen in the db/db mouse hearts. Addition of 600 μmol/l ßOHB to d_b/db_ mouse hearts perfused with 5 mmol/l glucose, 0.8 mmol/l palmitate, and 100 μU/ml insulin increased ketone oxidation rates, but did not decrease either glucose oxidation or fatty acid oxidation rates. The presence of ketones did not increase cardiac efficiency, but did increase ATP production rates, due to the additional contribution of ketone oxidation to energy production. The authors conclude that empagliflozin treatment is associated with an increase in ATP production, resulting in an enhanced energy status of the heart.
Keywords: ANOVA, analysis of variance; ATP, adenosine triphosphate; LV, left ventricular; PDH, pyruvate-dehydrogenase; cardiac efficiency; db/db; glucose oxidation; ketone oxidation; ßOHB, β-hydroxybutyrate.
Figures
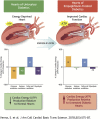
Graphical abstract
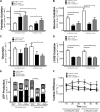
Figure 1
Body Weight, LV Mass, and Cardiac Function in Empagliflozin-Treated db/db Mice Body weight (n = 16 to 19/group) (A) and ventricular mass measured after sacrifice (n = 12 to 13/group) (B) are shown. Cardiac work presented in J ∙ min−1 ∙ gram dry weight−1**(C)** and cardiac output in ml ∙ min−1**(D)** (n = 19 to 20/group) determined by isolated working heart perfusion are shown. Data are presented as mean ± SEM. Data were analyzed by 1-way analysis of variance (ANOVA) followed by least significant difference (LSD) post hoc test. Cardiac work and cardiac output were analyzed by repeated 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. †p < 0.05 was considered as a significantly different comparison with db/db + Vehicle.
Figure 2
Absolute Metabolic Rates and Cardiac Efficiency in Ex Vivo Isolated Working Hearts From Empagliflozin-Treated db/db Mice Metabolic rates are shown for palmitate oxidation (n = 8 to 10/group) (A), glucose oxidation (n = 8 to 10/group) (B), glycolysis (8 to 9/group) (C), β-hydroxybutyrate (βOHB) oxidation (n = 8 to 9/group) (D), and total adenosine triphosphate (ATP) production (8 to 10/group) (E). Cardiac efficiency calculated as cardiac work/O2 consumed (n = 9 to 12/group) (F) is also shown. Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA to determine differences within the same insulin status and between groups in the presence or absence of insulin followed by LSD post hoc test. Four separate 1-way ANOVAs were performed to determine each substrate’s contribution to ATP production. Cardiac efficiency was analyzed by repeated 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. For E, ‡p < 0.05 was considered as a significantly different in comparison with C57BL/6J + Vehicle within the same insulin status. Abbreviations as in Figure 1.
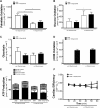
Figure 3
Cardiac Metabolic Rates, Energy Production, and Cardiac Efficiency in db/db Mouse Hearts Perfused With or Without 600 μmol/l βOHB Working heart perfusion derived palmitate (fatty acid) oxidation (n = 7 for db/db, n = 6 for db/db + 600 μM βOHB) (A), glucose oxidation (n = 5 for db/db, n = 8 for db/db + 600 μM βOHB) (B), and glycolysis (n = 4 to 5 for db/db, n = 7 to 8 for db/db + 600 μM βOHB) (C) levels. βOHB (ketone body) oxidation levels (n = 8 for db/db + 600 μM βOHB) (D) are also shown. Cardiac ATP production and comparison between contribution from glycolysis and the oxidation of glucose, palmitate, and βOHB (n = 5 to 8 for all groups) (E). Cardiac efficiency of the ex vivo heart as determined by normalizing cardiac work to oxygen consumption (n = 10 for db/db, n = 15 for db/db + 600 μM βOHB) (F). Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA followed by LSD post hoc test. Three separate 1-way ANOVAs were performed to determine each substrate’s contribution to ATP production. *p < 0.05 was considered as a significantly different comparison to the insulin-absent levels of the same group. For E, ‡p < 0.05 was considered as significantly different in comparison with the same group in the absence of insulin. Abbreviations as in Figures 1 and 2.
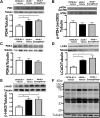
Figure 4
Cardiac Protein Expression and Regulation of Glucose and Fatty Acid Oxidation Enzymes and Its Regulation by Acetylation in Empagliflozin-Treated db/db Mice Cardiac protein expression of glucose oxidation enzyme pyruvate dehydrogenase (PDH) (n = 6–7/group) (A), phosphorylation of PDH at the serine 293 residue (n = 6 to 7/group) (B), protein expression of pyruvate dehydrogenase kinase 4 (PDK4) (n = 6 to 7/group) (C), fatty acid β-oxidation enzymes long chain acyl CoA dehydrogenase (LCAD) (D), and β-hydroxyacyl CoA dehydrogenase (β-HAD) (E) (n = 6 to 7/group) are shown. Total cardiac lysine acetylation blot (F) with quantification (G) (n = 7/group) along with lysine acetylation levels of PDH, LCAD, and β-HAD normalized to the anti-acetyllysine immunoglobulin heavy chain (n = 5 to 6/group) (H–J) are shown. Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. Abbreviations as Figures 1 and 2.
Figure 4
Cardiac Protein Expression and Regulation of Glucose and Fatty Acid Oxidation Enzymes and Its Regulation by Acetylation in Empagliflozin-Treated db/db Mice Cardiac protein expression of glucose oxidation enzyme pyruvate dehydrogenase (PDH) (n = 6–7/group) (A), phosphorylation of PDH at the serine 293 residue (n = 6 to 7/group) (B), protein expression of pyruvate dehydrogenase kinase 4 (PDK4) (n = 6 to 7/group) (C), fatty acid β-oxidation enzymes long chain acyl CoA dehydrogenase (LCAD) (D), and β-hydroxyacyl CoA dehydrogenase (β-HAD) (E) (n = 6 to 7/group) are shown. Total cardiac lysine acetylation blot (F) with quantification (G) (n = 7/group) along with lysine acetylation levels of PDH, LCAD, and β-HAD normalized to the anti-acetyllysine immunoglobulin heavy chain (n = 5 to 6/group) (H–J) are shown. Data are presented as mean ± SEM. Data were analyzed by 1-way ANOVA followed by LSD post hoc test. *p < 0.05 was considered as a significantly different comparison with C57BL/6J + Vehicle. Abbreviations as Figures 1 and 2.
Similar articles
- Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency.
Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD. Ho KL, et al. Cardiovasc Res. 2019 Sep 1;115(11):1606-1616. doi: 10.1093/cvr/cvz045. Cardiovasc Res. 2019. PMID: 30778524 Free PMC article. - Ketones provide an extra source of fuel for the failing heart without impairing glucose oxidation.
Pherwani S, Connolly D, Sun Q, Karwi QG, Carr M, Ho KL, Wagg CS, Zhang L, Levasseur J, Silver H, Dyck JRB, Lopaschuk GD. Pherwani S, et al. Metabolism. 2024 May;154:155818. doi: 10.1016/j.metabol.2024.155818. Epub 2024 Feb 17. Metabolism. 2024. PMID: 38369056 - Empagliflozin Decreases Lactate Generation in an NHE-1 Dependent Fashion and Increases α-Ketoglutarate Synthesis From Palmitate in Type II Diabetic Mouse Hearts.
Zhang H, Uthman L, Bakker D, Sari S, Chen S, Hollmann MW, Coronel R, Weber NC, Houten SM, van Weeghel M, Zuurbier CJ. Zhang H, et al. Front Cardiovasc Med. 2020 Dec 4;7:592233. doi: 10.3389/fcvm.2020.592233. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 33344518 Free PMC article. - Cardiac Energy Metabolism in Heart Failure.
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Lopaschuk GD, et al. Circ Res. 2021 May 14;128(10):1487-1513. doi: 10.1161/CIRCRESAHA.121.318241. Epub 2021 May 13. Circ Res. 2021. PMID: 33983836 Free PMC article. Review. - Empagliflozin Prevents Worsening of Cardiac Function in an Experimental Model of Pressure Overload-Induced Heart Failure.
Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G, Fedak PWM, Verma S, Dyck JRB. Byrne NJ, et al. JACC Basic Transl Sci. 2017 Aug 4;2(4):347-354. doi: 10.1016/j.jacbts.2017.07.003. eCollection 2017 Aug. JACC Basic Transl Sci. 2017. PMID: 30062155 Free PMC article. Review.
Cited by
- Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Guardians against Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Heart Diseases.
Pham LTT, Mangmool S, Parichatikanond W. Pham LTT, et al. ACS Pharmacol Transl Sci. 2024 Oct 16;7(11):3279-3298. doi: 10.1021/acsptsci.4c00240. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539254 Review. - Sodium glucose cotransporter 2 inhibitors in the management of heart failure: Veni, Vidi, and Vici.
Bhandari M, Pradhan A, Vishwakarma P, Singh A, Sethi R. Bhandari M, et al. World J Cardiol. 2024 Oct 26;16(10):550-563. doi: 10.4330/wjc.v16.i10.550. World J Cardiol. 2024. PMID: 39492976 Free PMC article. Review. - Efficacy of Sodium-Glucose 2 Transporter Inhibitors in Heart Failure With Preserved Ejection Fraction: A Narrative Review.
Maged R, Sinha M, Koneru HM, Sarwar H, Bandi VV, Tarar P, Halawa N. Maged R, et al. Cureus. 2024 Sep 17;16(9):e69623. doi: 10.7759/cureus.69623. eCollection 2024 Sep. Cureus. 2024. PMID: 39429273 Free PMC article. Review. - Haemodynamic Effects of Sodium-Glucose Cotransporter 2 Inhibitor Treatment in Chronic Heart Failure Patients.
Nilsson CN, Ersbøll MK, Gustafsson F. Nilsson CN, et al. Card Fail Rev. 2024 Sep 11;10:e09. doi: 10.15420/cfr.2023.25. eCollection 2024. Card Fail Rev. 2024. PMID: 39309522 Free PMC article. Review. - Advances in understanding and managing pediatric heart failure and transplant.
Xu W, Richmond M. Xu W, et al. Curr Opin Pediatr. 2024 Oct 1;36(5):489-495. doi: 10.1097/MOP.0000000000001393. Epub 2024 Aug 12. Curr Opin Pediatr. 2024. PMID: 39254752 Review.
References
- Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. - PubMed
- Neal B., Perkovic V., Mahaffey K.W. CANVAS Program Collaborative Group, Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. - PubMed
- Farkouh M.E., Verma S. Prevention of heart failure with SGLT-2 inhibition: insights from CVD-REAL. J Am Coll Cardiol. 2018;71:2507–2510. - PubMed
- Cavender M.A., Norhammar A., Birkeland K.I., CVD-REAL Investigators and Study Group SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018;71:2497–2506. - PubMed
- Verma S., Mazer C.D., Al-Omran M. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous