A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer's disease: A pilot study - PubMed (original) (raw)
A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer's disease: A pilot study
Carolyn W Zhu et al. Alzheimers Dement (N Y). 2018.
Abstract
Introduction: Human studies on low-dose resveratrol are scarce. This study aims to evaluate the safety, tolerability, and efficacy of an oral preparation of resveratrol, glucose, and malate (RGM) in slowing the progression of Alzheimer's disease (AD).
Methods: Thirty-nine subjects with mild to moderate AD who were free of life-threatening disease and who did not have contraindications to the use of the study product were screened. Progression of AD was measured by change in the cognitive portion of the Alzheimer's Disease Assessment Scale-cognitive subscale. Secondary outcomes included Clinician's Global Impression of Change, Mini-Mental State Examination, Alzheimer's Disease Cooperative Study-Activities of Daily Living Scale, and Neuropsychiatric Inventory. 15 mL of the following preparation per dose, i.e., 5 g dextrose, 5 g malate, and 5 mg resveratrol, or matching placebo was ingested with an 8 oz glass of commercial unsweetened grape juice twice a day for 1 year. Group differences in the rate of change in the outcome measures were examined using generalized estimating equations.
Results: The treatment and control groups were similar on all of the screening variables. At 12 months, change scores on Alzheimer's Disease Assessment Scale-cognitive subscale, Mini-Mental State Examination, Alzheimer's Disease Cooperative Study-Activities of Daily Living Scale, or Neuropsychiatric Inventory all showed less deterioration in the treatment than the control group; however, none of the change scores reached statistical significance. The most common AE were falls, all in the control group. None of the falls were deemed to be study related.
Conclusion: Low-dose oral resveratrol is safe and well tolerated. Interpretation of the effects on clinical outcomes trajectories remains uncertain. A larger study is required to determine whether low-dose resveratrol may be beneficial.
Trial registration: ClinicalTrials.gov (NCT00678431), Registered 05/15/2008.
Keywords: Double-blind methods; Drug therapy; Efficacy; Resveratrol; Safety.
Figures
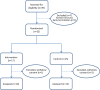
Fig. 1
Consort Diagram and Disposition by Treatment Group.
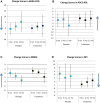
Fig. 2
Mean change scores from baseline at each follow-up visit in treatment and placebo groups. Vertical bars represent standard deviation. Blue arrow indicates direction of improvement. (A) Positive change scores in ADAS-cog indicate worsening impairment from baseline. (B) Positive change scores in ADCS-ADL indicate improvement from baseline. (C) Positive change scores in MMSE indicate improvement from baseline. (D) Positive change scores in NPI indicate worsening impairment from baseline. Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale–cognitive subscale; MMSE, Mini–Mental State Examination; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale; NPI, Neuropsychiatric Inventory.
Similar articles
- Rivastigmine for Alzheimer's disease.
Birks JS, Grimley Evans J. Birks JS, et al. Cochrane Database Syst Rev. 2015 Apr 10;(4):CD001191. doi: 10.1002/14651858.CD001191.pub3. Cochrane Database Syst Rev. 2015. PMID: 25858345 Review. - A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer's disease.
Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, Kaufman AC, Rosenberg BJ, Sekine-Konno T, Varma P, Chen K, Koleske AJ, Reiman EM, Strittmatter SM, van Dyck CH. Nygaard HB, et al. Alzheimers Res Ther. 2015 Apr 14;7(1):35. doi: 10.1186/s13195-015-0119-0. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 25874001 Free PMC article. - Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer's disease.
Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, Gulanski B. Morris JC, et al. Neurology. 1998 May;50(5):1222-30. doi: 10.1212/wnl.50.5.1222. Neurology. 1998. PMID: 9595967 Clinical Trial. - Effects of Resveratrol Supplementation on the Cognitive Function of Patients with Alzheimer's Disease: A Systematic Review of Randomized Controlled Trials.
Tosatti JAG, Fontes AFDS, Caramelli P, Gomes KB. Tosatti JAG, et al. Drugs Aging. 2022 Apr;39(4):285-295. doi: 10.1007/s40266-022-00923-4. Epub 2022 Feb 21. Drugs Aging. 2022. PMID: 35187615
Cited by
- Redox signaling and Alzheimer's disease: from pathomechanism insights to biomarker discovery and therapy strategy.
Chen YY, Wang MC, Wang YN, Hu HH, Liu QQ, Liu HJ, Zhao YY. Chen YY, et al. Biomark Res. 2020 Sep 11;8:42. doi: 10.1186/s40364-020-00218-z. eCollection 2020. Biomark Res. 2020. PMID: 32944245 Free PMC article. Review. - Natural Products Targeting Amyloid Beta in Alzheimer's Disease.
Lee JH, Ahn NH, Choi SB, Kwon Y, Yang SH. Lee JH, et al. Int J Mol Sci. 2021 Feb 26;22(5):2341. doi: 10.3390/ijms22052341. Int J Mol Sci. 2021. PMID: 33652858 Free PMC article. Review. - Mitochondrial Dysfunction in Alzheimer's Disease: Opportunities for Drug Development.
Bhatia S, Rawal R, Sharma P, Singh T, Singh M, Singh V. Bhatia S, et al. Curr Neuropharmacol. 2022;20(4):675-692. doi: 10.2174/1570159X19666210517114016. Curr Neuropharmacol. 2022. PMID: 33998995 Free PMC article. - Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer's Disease.
Plascencia-Villa G, Perry G. Plascencia-Villa G, et al. Antioxidants (Basel). 2023 Aug 17;12(8):1628. doi: 10.3390/antiox12081628. Antioxidants (Basel). 2023. PMID: 37627623 Free PMC article. Review. - Evidence of Clinical Efficacy and Pharmacological Mechanisms of Resveratrol in the Treatment of Alzheimer's Disease.
Jin S, Guan X, Min D. Jin S, et al. Curr Alzheimer Res. 2023;20(8):588-602. doi: 10.2174/0115672050272577231120060909. Curr Alzheimer Res. 2023. PMID: 38047366 Free PMC article.
References
- Citron M. Strategies for disease modification in Alzheimer's disease. Nat Rev Neurosci. 2004;5:677–685. - PubMed
- Reisberg B., Doody R., Stoffler A., Schmitt F., Ferris S., Mobius H.J. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. - PubMed
- Hock C., Konietzko U., Streffer J.R., Tracy J., Signorell A., Muller-Tillmanns B. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources