Prion Protein PRNP: A New Player in Innate Immunity? The Aβ Connection - PubMed (original) (raw)
Prion Protein PRNP: A New Player in Innate Immunity? The Aβ Connection
Richard Lathe et al. J Alzheimers Dis Rep. 2017.
Abstract
The prion protein PRNP has been centrally implicated in the transmissible spongiform encephalopathies (TSEs), but its normal physiological role remains obscure. We highlight emerging evidence that PRNP displays antimicrobial activity, inhibiting the replication of multiple viruses, and also interacts directly with Alzheimer's disease (AD) amyloid-β (Aβ) peptide whose own antimicrobial role is now increasingly secure. PRNP and Aβ share share membrane-penetrating, nucleic acid binding, and antiviral properties with classical antimicrobial peptides such as LL-37. We discuss findings that binding of abnormal nucleic acids to PRNP leads to oligomerization of the protein, and suggest that this may be an entrapment and sequestration process that contributes to its antimicrobial activity. Some antimicrobial peptides are known to be exploited by infectious agents, and we cover evidence that PRNP is usurped by herpes simplex virus (HSV-1) that has evolved a virus-encoded 'anti-PRNP'.unction. These findings suggest that PRNP, like LL-37 and Aβ, is likely to be a component of the innate immune system, with implications for the pathoetiology of both AD and TSE.
Keywords: Alzheimer’s disease; PRNP; amyloid-β peptide; herpes simplex; innate immunity; spongiform encephalopathy.
Figures
Fig.1
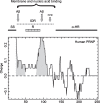
Domains of Human PRNP Protein. The graph plots the charge of the mature protein (residues 23–253) analyzed with EMBOSS (
http://www.bioinformatics.nl/cgi-bin/emboss/charge
; windowsize = 20). Grey, basic regions. Abbreviations: Aβ, binding sites for Aβ peptide; FPR, formyl peptide receptor binding site; α-HR, α-helical region; IDR, intrinsically disordered region; R, octapeptide repeats; SS, signal sequence.
Fig.2
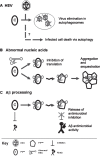
Antimicrobial Activities of PRNP. In addition to the listed categories, other mechanisms are likely to include the generation of reactive oxygen species, and binding to immunomodulatory molecules including APOE and formyl peptide receptors is likely to direct the recruitment of other actors in innate immunity. Direct binding to Aβ (not depicted) and potential interactions with other antimicrobial peptides that bind to Aβ (e.g., LL-37, α-synuclein) add a further dimension. Abbreviation: HSV, herpes simplex virus type 1.
Similar articles
- The antimicrobial protection hypothesis of Alzheimer's disease.
Moir RD, Lathe R, Tanzi RE. Moir RD, et al. Alzheimers Dement. 2018 Dec;14(12):1602-1614. doi: 10.1016/j.jalz.2018.06.3040. Epub 2018 Oct 9. Alzheimers Dement. 2018. PMID: 30314800 - β-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1.
Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, Fülöp T Jr. Bourgade K, et al. Biogerontology. 2015 Feb;16(1):85-98. doi: 10.1007/s10522-014-9538-8. Epub 2014 Nov 7. Biogerontology. 2015. PMID: 25376108 - In vitro prion protein conversion suggests risk of bighorn sheep (Ovis canadensis) to transmissible spongiform encephalopathies.
Morawski AR, Carlson CM, Chang H, Johnson CJ. Morawski AR, et al. BMC Vet Res. 2013 Aug 9;9:157. doi: 10.1186/1746-6148-9-157. BMC Vet Res. 2013. PMID: 23938169 Free PMC article. - The use of transgenic mice in the investigation of transmissible spongiform encephalopathies.
Weissmann C, Fischer M, Raeber A, Büeler H, Sailer A, Shmerling D, Rülicke T, Brandner S, Aguzzi A. Weissmann C, et al. Rev Sci Tech. 1998 Apr;17(1):278-90. doi: 10.20506/rst.17.1.1079. Rev Sci Tech. 1998. PMID: 9638817 Review.
Cited by
- Neuroprotective Effects of Curcumin in Neurodegenerative Diseases.
Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. Genchi G, et al. Foods. 2024 Jun 5;13(11):1774. doi: 10.3390/foods13111774. Foods. 2024. PMID: 38891002 Free PMC article. Review. - Zinc and Copper Ions Differentially Regulate Prion-Like Phase Separation Dynamics of Pan-Virus Nucleocapsid Biomolecular Condensates.
Monette A, Mouland AJ. Monette A, et al. Viruses. 2020 Oct 18;12(10):1179. doi: 10.3390/v12101179. Viruses. 2020. PMID: 33081049 Free PMC article. - Virulent Pseudomonas aeruginosa infection converts antimicrobial amyloids into cytotoxic prions.
Voth S, Gwin M, Francis CM, Balczon R, Frank DW, Pittet JF, Wagener BM, Moser SA, Alexeyev M, Housley N, Audia JP, Piechocki S, Madera K, Simmons A, Crawford M, Stevens T. Voth S, et al. FASEB J. 2020 Jul;34(7):9156-9179. doi: 10.1096/fj.202000051RRR. Epub 2020 May 15. FASEB J. 2020. PMID: 32413239 Free PMC article. - Prion protein PrP nucleic acid binding and mobilization implicates retroelements as the replicative component of transmissible spongiform encephalopathy.
Lathe R, Darlix JL. Lathe R, et al. Arch Virol. 2020 Mar;165(3):535-556. doi: 10.1007/s00705-020-04529-2. Epub 2020 Feb 5. Arch Virol. 2020. PMID: 32025859 Free PMC article. Review.
References
- Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, Fulop T Jr (2015) Beta-amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 16, 85–98. - PubMed
- Bourgade K, Le PA, Bocti C, Witkowski JM, Dupuis G, Frost EH, Fulop T Jr (2016) Protective effect of amyloid-beta peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J Alzheimers Dis 50, 1227–1241. - PubMed
LinkOut - more resources
Full Text Sources