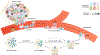Tumour heterogeneity and metastasis at single-cell resolution - PubMed (original) (raw)
Review
Tumour heterogeneity and metastasis at single-cell resolution
Devon A Lawson et al. Nat Cell Biol. 2018 Dec.
Abstract
Tumours comprise a heterogeneous collection of cells with distinct genetic and phenotypic properties that can differentially promote progression, metastasis and drug resistance. Emerging single-cell technologies provide a new opportunity to profile individual cells within tumours and investigate what roles they play in these processes. This Review discusses key technological considerations for single-cell studies in cancer, new findings using single-cell technologies and critical open questions for future applications.
Figures
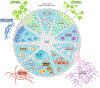
Fig. 1 ∣. Common types of intratumour heterogeneity and its regulation by intrinsic and extrinsic factors.
Tumours comprise a heterogeneous population of cells, which is regulated by both intrinsic and extrinsic factors. Tumour cells vary in biomarker expression, epigenetic landscape, hypoxic state, metabolic state, stage of differentiation, invasive potential and genotype due to genomic instability. The tumour microenvironment can also be heterogeneous, in which different types of fibroblasts, pro-tumour and anti-tumour immune infiltrate, vascular and lymphatic vessel density and extracellular matrix (ECM) composition affect tumour cell heterogeneity and function.
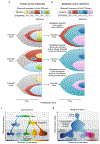
Fig. 2 ∣. Deciphering subclonal composition and cell types and states in single-cell omics data.
a, Inferring clonal trajectories and subclonal heterogeneity from bulk primary tumour genome sequencing data. In this example experiment, a tumour is sampled at a single time point (dotted lines). The table shows the frequency of each detected mutation. The panels show three (of many) possible clonal trajectories that can be inferred. The nodes represent points at which a mutation occurred, and overlapping coloured regions indicate that each of the mutations is present within any cell that is part of that population. b, Challenges associated with deciphering the genotype of a metastatic founder clone and subclonal trajectories from bulk genome sequencing of paired metastatic and primary tumours. The table shows the observed mutation frequencies in an example experiment in which a metastatic tumour from the individual in a was sequenced. The panels show three possible explanations for the observed frequencies. c, Cell types and states found in normal tissues. Tissues comprise different mature ‘cell types’ (labelled 1–5), which carry out specified functions. Cells within a ‘type’ can exist in a spectrum of allowable ‘cell states’ depending on the physiological status of the tissue. Mature cell types are derived from stem cells through a series of discrete differentiation intermediates or progenitors. The circles represent single cells, and the colour clouds represent the spectrum of allowable states. The density of circles represents the probability of observing a cell with that phenotype in a scRNA-seq experiment. d, Tumour cell types and states differ from normal tissue. Single-cell studies have shown that tumours contain stem-like cells (CSCs) and that differentiation is often noisy, skewed towards specific cell lineages.
Fig. 3 ∣. Genetic and phenotypic properties of metastasis-initiating cells at the single-cell level.
Metastasis is a rare event, in which most cancer cells cannot progress through major bottlenecks associated with invasion, intravasation, extravasation, seeding and colonization to produce a malignant macrometastatic tumour. In this model, cancer cells are heterogeneous in genotype (nuclei) and phenotype (cytoplasm), and metastasis-initiating cells possess a distinct combination of both. Dashed arrow indicates that cancer cells within micrometastases can die. Death rates within micrometastases can balance proliferation rates, and thereby prevent progression to macrometastasis by the failure to produce net positive growth.
Similar articles
- Tumour Heterogeneity: The Key Advantages of Single-Cell Analysis.
Tellez-Gabriel M, Ory B, Lamoureux F, Heymann MF, Heymann D. Tellez-Gabriel M, et al. Int J Mol Sci. 2016 Dec 20;17(12):2142. doi: 10.3390/ijms17122142. Int J Mol Sci. 2016. PMID: 27999407 Free PMC article. Review. - Single-cell analysis on stromal fibroblasts in the microenvironment of solid tumours.
Musa M. Musa M. Adv Med Sci. 2020 Mar;65(1):163-169. doi: 10.1016/j.advms.2019.12.001. Epub 2020 Jan 20. Adv Med Sci. 2020. PMID: 31972467 Review. - Tumor Functional Heterogeneity Unraveled by scRNA-seq Technologies.
González-Silva L, Quevedo L, Varela I. González-Silva L, et al. Trends Cancer. 2020 Jan;6(1):13-19. doi: 10.1016/j.trecan.2019.11.010. Epub 2020 Jan 3. Trends Cancer. 2020. PMID: 31952776 Review. - Advances in understanding tumour evolution through single-cell sequencing.
Kuipers J, Jahn K, Beerenwinkel N. Kuipers J, et al. Biochim Biophys Acta Rev Cancer. 2017 Apr;1867(2):127-138. doi: 10.1016/j.bbcan.2017.02.001. Epub 2017 Feb 11. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28193548 Free PMC article. Review. - Genetic Heterogeneity of Single Circulating Tumour Cells in Colorectal Carcinoma.
Hamid FB, Gopalan V, Matos M, Lu CT, Lam AK. Hamid FB, et al. Int J Mol Sci. 2020 Oct 20;21(20):7766. doi: 10.3390/ijms21207766. Int J Mol Sci. 2020. PMID: 33092235 Free PMC article.
Cited by
- Deciphering cellular plasticity in pancreatic cancer for effective treatments.
Uddin MH, Zhang D, Muqbil I, El-Rayes BF, Chen H, Philip PA, Azmi AS. Uddin MH, et al. Cancer Metastasis Rev. 2024 Mar;43(1):393-408. doi: 10.1007/s10555-023-10164-5. Epub 2024 Jan 9. Cancer Metastasis Rev. 2024. PMID: 38194153 Review. - Organ-Specificity of Breast Cancer Metastasis.
Ibragimova MK, Tsyganov MM, Kravtsova EA, Tsydenova IA, Litviakov NV. Ibragimova MK, et al. Int J Mol Sci. 2023 Oct 26;24(21):15625. doi: 10.3390/ijms242115625. Int J Mol Sci. 2023. PMID: 37958607 Free PMC article. Review. - Does Cancer Biology Rely on Parrondo's Principles?
Capp JP, Nedelcu AM, Dujon AM, Roche B, Catania F, Ujvari B, Alix-Panabières C, Thomas F. Capp JP, et al. Cancers (Basel). 2021 May 3;13(9):2197. doi: 10.3390/cancers13092197. Cancers (Basel). 2021. PMID: 34063648 Free PMC article. Review. - Proteomics and liquid biopsy characterization of human EMT-related metastasis in colorectal cancer.
Huang MS, Fu LH, Yan HC, Cheng LY, Ru HM, Mo S, Wei CY, Li DM, Mo XW, Tang WZ, Yan LH. Huang MS, et al. Front Oncol. 2022 Sep 28;12:790096. doi: 10.3389/fonc.2022.790096. eCollection 2022. Front Oncol. 2022. PMID: 36249004 Free PMC article. - Exploring Therapeutic Avenues in Lung Cancer: The Epigenetic Perspective.
Munteanu R, Tomuleasa C, Iuga CA, Gulei D, Ciuleanu TE. Munteanu R, et al. Cancers (Basel). 2023 Nov 13;15(22):5394. doi: 10.3390/cancers15225394. Cancers (Basel). 2023. PMID: 38001653 Free PMC article. Review.
References
- Heppner GH Tumor heterogeneity. Cancer Res. 44, 2259–2265 (1984). - PubMed
- Marusyk A, Almendro V & Polyak K Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 EB009418/EB/NIBIB NIH HHS/United States
- T32 CA009054/CA/NCI NIH HHS/United States
- U01 CA199315/CA/NCI NIH HHS/United States
- K22 CA190511/CA/NCI NIH HHS/United States
- U54 CA217378/CA/NCI NIH HHS/United States
- R00 CA181490/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources