Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis - PubMed (original) (raw)
Meta-Analysis
Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis
Joanna C Crocker et al. BMJ. 2018.
Abstract
Objective: To investigate the impact of patient and public involvement (PPI) on rates of enrolment and retention in clinical trials and explore how this varies with the context and nature of PPI.
Design: Systematic review and meta-analysis.
Data sources: Ten electronic databases, including Medline, INVOLVE Evidence Library, and clinical trial registries.
Eligibility criteria: Experimental and observational studies quantitatively evaluating the impact of a PPI intervention, compared with no intervention or non-PPI intervention(s), on participant enrolment and/or retention rates in a clinical trial or trials. PPI interventions could include additional non-PPI components inseparable from the PPI (for example, other stakeholder involvement).
Data extraction and analysis: Two independent reviewers extracted data on enrolment and retention rates, as well as on the context and characteristics of PPI intervention, and assessed risk of bias. Random effects meta-analyses were used to determine the average effect of PPI interventions on enrolment and retention in clinical trials: main analysis including randomised studies only, secondary analysis adding non-randomised studies, and several exploratory subgroup and sensitivity analyses.
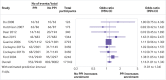
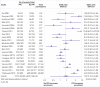
Results: 26 studies were included in the review; 19 were eligible for enrolment meta-analysis and five for retention meta-analysis. Various PPI interventions were identified with different degrees of involvement, different numbers and types of people involved, and input at different stages of the trial process. On average, PPI interventions modestly but significantly increased the odds of participant enrolment in the main analysis (odds ratio 1.16, 95% confidence interval and prediction interval 1.01 to 1.34). Non-PPI components of interventions may have contributed to this effect. In exploratory subgroup analyses, the involvement of people with lived experience of the condition under study was significantly associated with improved enrolment (odds ratio 3.14 v 1.07; P=0.02). The findings for retention were inconclusive owing to the paucity of eligible studies (odds ratio 1.16, 95% confidence interval 0.33 to 4.14), for main analysis).
Conclusions: These findings add weight to the case for PPI in clinical trials by indicating that it is likely to improve enrolment of participants, especially if it includes people with lived experience of the health condition under study. Further research is needed to assess which types of PPI work best in particular contexts, the cost effectiveness of PPI, the impact of PPI at earlier stages of trial design, and the impact of PPI interventions specifically targeting retention.
Systematic review registration: PROSPERO CRD42016043808.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work other than that described above; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
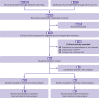
Fig 1
PRISMA flow diagram of records/studies included at each stage of screening and in final meta-analyses. PPI=patient and public involvement
Fig 2
Odds ratios for patient enrolment in clinical trial with versus without patient and public involvement (PPI) intervention (randomised studies only)
Fig 3
Odds ratios for patient enrolment in clinical trial with patient and public involvement (PPI) intervention versus no PPI or non-PPI intervention (randomised and non-randomised studies combined)
Similar articles
- The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - When participants get involved: reconsidering patient and public involvement in clinical trials at the MRC Clinical Trials Unit at UCL.
Vale CL, Cragg WJ, Cromarty B, Hanley B, South A, Stephens R, Sturgeon K, Gafos M. Vale CL, et al. Trials. 2018 Feb 7;19(1):95. doi: 10.1186/s13063-018-2471-4. Trials. 2018. PMID: 29415751 Free PMC article. - Psychological interventions to improve self-management of type 1 and type 2 diabetes: a systematic review.
Winkley K, Upsher R, Stahl D, Pollard D, Kasera A, Brennan A, Heller S, Ismail K. Winkley K, et al. Health Technol Assess. 2020 Jun;24(28):1-232. doi: 10.3310/hta24280. Health Technol Assess. 2020. PMID: 32568666 Free PMC article. - Audio-visual presentation of information for informed consent for participation in clinical trials.
Ryan RE, Prictor MJ, McLaughlin KJ, Hill SJ. Ryan RE, et al. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD003717. doi: 10.1002/14651858.CD003717.pub2. Cochrane Database Syst Rev. 2008. PMID: 18254029 Updated. Review. - Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis.
Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, Price MJ, Bates EJ, Turner AM, Bayliss S, Moore D, Singh S, Adab P, Fitzmaurice DA, Jowett S, Jolly K. Jordan RE, et al. Health Technol Assess. 2015 May;19(36):1-516. doi: 10.3310/hta19360. Health Technol Assess. 2015. PMID: 25980984 Free PMC article. Review.
Cited by
- A comparative study to elucidate factors explaining willingness to use home-care robots in Japan, Ireland, and Finland.
Ide H, Suwa S, Akuta Y, Kodate N, Tsujimura M, Ishimaru M, Shimamura A, Kitinoja H, Donnelly S, Hallila J, Toivonen M, Bergman-Kärpijoki C, Takahashi E, Yu W. Ide H, et al. Sci Rep. 2024 Nov 12;14(1):27656. doi: 10.1038/s41598-024-79414-y. Sci Rep. 2024. PMID: 39533068 Free PMC article. - Leveraging Social Determinants of Health to Enhance Recruitment of Underrepresented Populations in Clinical Trials.
King S, Trabanino S, Azizi Z, Rodriguez F. King S, et al. Methodist Debakey Cardiovasc J. 2024 Nov 5;20(5):81-88. doi: 10.14797/mdcvj.1447. eCollection 2024. Methodist Debakey Cardiovasc J. 2024. PMID: 39525382 Free PMC article. Review. - Community-Engaged Approaches for Improving the Inclusion of Diverse Communities in a Nutrition Clinical Trial.
Adeyemo MA, Trinh J, Perez D, Bozeman E, Ntekume E, Gardner J, Thames G, Luong T, Carson SL, Vassar S, Norris K, Li Z, Brown AF, Casillas A. Adeyemo MA, et al. Nutrients. 2024 Oct 23;16(21):3592. doi: 10.3390/nu16213592. Nutrients. 2024. PMID: 39519425 Free PMC article. - Training and peer-group coaching for pairs of researchers and patient representatives to support continuous two-way learning.
Schoemaker CG, Schalkers I, de Jong BA, Wissink W, le Loux S, Buijsen RAM, Russcher K, van der Steeg FAM, Blom J, Vroonland E. Schoemaker CG, et al. Res Involv Engagem. 2024 Oct 25;10(1):110. doi: 10.1186/s40900-024-00646-3. Res Involv Engagem. 2024. PMID: 39456103 Free PMC article. - Beyond effectiveness in eHealth trials: Process evaluation of a stepped-care programme to support healthcare workers with psychological distress (RESPOND-HCWs).
Mediavilla R, García-Vázquez B, McGreevy KR, Underhill J, Bayón C, Bravo-Ortiz MF, Muñoz-Sanjosé A, Haro JM, Monistrol-Mula A, Nicaise P, Petri-Romão P, McDaid D, Park AL, Melchior M, Vuillermoz C, Turrini G, Compri B, Purgato M, Roos R, Witteveen AB, Sijbrandij M, Bryant RA, Fuhr D, Ayuso-Mateos JL. Mediavilla R, et al. Digit Health. 2024 Oct 18;10:20552076241287678. doi: 10.1177/20552076241287678. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 39430699 Free PMC article.
References
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7:e015276. 10.1136/bmjopen-2016-015276 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical