Evaluating the Impacts of Methylsulfonylmethane on Allergic Rhinitis After a Standard Allergen Challenge: Randomized Double-Blind Exploratory Study - PubMed (original) (raw)
Evaluating the Impacts of Methylsulfonylmethane on Allergic Rhinitis After a Standard Allergen Challenge: Randomized Double-Blind Exploratory Study
Susan Hewlings et al. JMIR Res Protoc. 2018.
Abstract
Background: The sulfur-containing compound methylsulfonylmethane (MSM) has been used as a dietary supplement for a variety of reported health benefits. Clinical observations and case studies have indicated that MSM may help alleviate allergic rhinitis; however, this effect has not been evaluated under controlled conditions.
Objective: This study aimed to determine the effects of MSM consumption on allergic rhinitis symptoms after provocation with a standardized allergen.
Methods: We recruited healthy participants with a history of allergic nasal congestion to participate in a randomized, double-blind, adaptive-design study. Participants were administered a standardized allergen in clinic to determine the presence or absence of an allergic response. Participant responses were recorded using a recognized measure of nasal patency, peak nasal inspiratory flow (PNIF), and by a visual analog scale to score the severity of their allergy-related nasal symptoms. After we collected baseline nasal responses to allergen, followed by a 1-week washout period, participants returned to the clinic and were exposed to allergen after taking an acute high dose of 12 g of MSM. We then randomly assigned participants to a lower dose of MSM (1 g, 3 g, or 6 g), which they consumed once a day for 14 days. Participants returned to the clinic for repeat assessments while again taking their assigned daily dose of MSM.
Results: All MSM treatment courses significantly reduced visual analog scale average nasal symptoms in a longitudinal comparison across all participants, with low-dose treatments decreasing symptoms by 53.72% (P=.001), and an acute 12-g dose decreasing symptoms by 22.49% (P=.03). Although the acute dose of MSM did not yield significant changes in nasal patency, low "everyday" doses significantly relieved nasal obstruction as indicated by a 17.32% (P=.02) increase in PNIF across all participants. The most effective dose across all measurements was daily consumption of 3 g of MSM, which significantly decreased all nasal symptoms (nasal obstruction, rhinorrhea, watery or itchy eyes and nose, and sneezing) and further was found to significantly (P=.01) increase PNIF.
Conclusions: The MSM study product provided significant relief of allergic rhinitis symptoms and objective nasal obstruction measurements without the occurrence of adverse events. Oral consumption of the study product may reduce the symptoms and onset of allergic rhinitis without the side effects associated with standard-care medication.
Trial registration: ClinicalTrials.gov NCT02342483; https://clinicaltrials.gov/ct2/show/NCT02342483 (Archived by WebCite at http://www.webcitation.org/73vLKNvAp).
Keywords: allergic rhinitis; allergies; methylsulfonylmethane; sulfur; supplements.
©Susan Hewlings, Douglas S Kalman. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 29.11.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Figure 1
Attrition chart for the study. MSM: methylsulfonylmethane; V1: visit 1, screening visit; V2: visit 2; V3: visit 3.
Figure 2
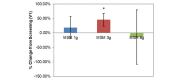
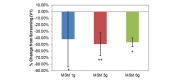
Methylsulfonylmethane (MSM) 12g percent change from screening. Peak nasal inspiratory flow (PNIF) and visual analog scale (VAS) nasal scores show the effects of an acute high methylsulfonylmethane dose (12 g) on patient symptoms. Significance was tested using linear mixed model analysis. *P ≤.05; **P ≤.01.
Figure 3
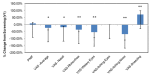
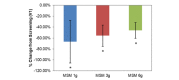
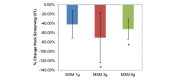
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in peak nasal inspiratory flow from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05.
Figure 4
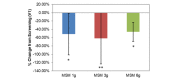
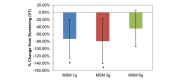
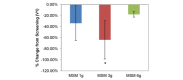
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale average nasal symptoms from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05; **P ≤.01.
Figure 5
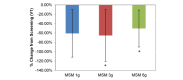
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale nasal obstruction from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05; **P ≤.01.
Figure 6
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale rhinorrhea from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05.
Figure 7
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale watery eyes from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05.
Figure 8
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale itching eyes from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05.
Figure 9
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale itching nose from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05.
Figure 10
Comparison between 3 subchronic doses of methylsulfonylmethane (MSM; 1 g, 3 g, and 6 g): percentage change in visual analog scale sneezing from V1 (screening) to V3 (day 14). Significance was tested using linear mixed model analysis. *P ≤.05.
Similar articles
- A proprietary blend of quail egg for the attenuation of nasal provocation with a standardized allergenic challenge: a randomized, double-blind, placebo-controlled study.
Benichou AC, Armanet M, Bussière A, Chevreau N, Cardot JM, Tétard J. Benichou AC, et al. Food Sci Nutr. 2014 Nov;2(6):655-63. doi: 10.1002/fsn3.147. Epub 2014 Jul 20. Food Sci Nutr. 2014. PMID: 25493182 Free PMC article. - Efficacy of a Quail Eggs-Based Dietary Supplement for Allergic Rhinitis: Results of a Single-Arm Trial.
Syrigou E, Psarros F, Makris M, Grapsa D, Syrigos K. Syrigou E, et al. J Diet Suppl. 2021;18(1):17-30. doi: 10.1080/19390211.2019.1694121. Epub 2019 Nov 25. J Diet Suppl. 2021. PMID: 31762363 Clinical Trial. - [Rhinitis in adults].
Kalogjera L. Kalogjera L. Acta Med Croatica. 2011;65(2):181-7. Acta Med Croatica. 2011. PMID: 22359885 Review. Croatian. - Clinical trial design, nasal allergen challenge models, and considerations of relevance to pediatrics, nasal polyposis, and different classes of medication.
Akerlund A, Andersson M, Leflein J, Lildholdt T, Mygind N. Akerlund A, et al. J Allergy Clin Immunol. 2005 Mar;115(3 Suppl 1):S460-82. doi: 10.1016/j.jaci.2004.12.016. J Allergy Clin Immunol. 2005. PMID: 15746883 Review.
Cited by
- New Insight into Drugs to Alleviate Atopic March via Network Pharmacology-Based Analysis.
Oh KK, Adnan M, Cho DH. Oh KK, et al. Curr Issues Mol Biol. 2022 May 18;44(5):2257-2274. doi: 10.3390/cimb44050153. Curr Issues Mol Biol. 2022. PMID: 35678682 Free PMC article.
References
- Small P, Frenkiel S, Becker A, Boisvert P, Bouchard J, Carr S, Cockcroft D, Denburg J, Desrosiers M, Gall R, Hamid Q, Hébert J, Javer A, Keith P, Kim H, Lavigne F, Lemiàre C, Massoud E, Payton K, Schellenberg B, Sussman G, Tannenbaum D, Watson W, Witterick I, Wright E. Rhinitis: a practical and comprehensive approach to assessment and therapy. J Otolaryngol. 2007;36(S1):S5–S27. doi: 10.2310/7070.2006.X002. - DOI
- Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. 2011 Jul;3(3):148–56. doi: 10.4168/aair.2011.3.3.148. https://e-aair.org/DOIx.php?id=10.4168/aair.2011.3.3.148 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources