The inflammatory function of human IgA - PubMed (original) (raw)
Review
The inflammatory function of human IgA
Ivo S Hansen et al. Cell Mol Life Sci. 2019 Mar.
Abstract
The prevailing concept regarding the immunological function of immunoglobulin A (IgA) is that it binds to and neutralizes pathogens to prevent infection at mucosal sites of the body. However, recently, it has become clear that in humans IgA is also able to actively contribute to the initiation of inflammation, both at mucosal and non-mucosal sites. This additional function of IgA is initiated by the formation of immune complexes, which trigger Fc alpha Receptor I (FcαRI) to synergize with various other receptors to amplify inflammatory responses. Recent findings have demonstrated that co-stimulation of FcαRI strongly affects pro-inflammatory cytokine production by various myeloid cells, including different dendritic cell subsets, macrophages, monocytes, and Kupffer cells. FcαRI-induced inflammation plays a crucial role in orchestrating human host defense against pathogens, as well as the generation of tissue-specific immunity. In addition, FcαRI-induced inflammation is suggested to be involved in the pathogenesis of various chronic inflammatory disorders, including inflammatory bowel disease, celiac disease, and rheumatoid arthritis. Combined, IgA-induced inflammation may be used to either promote inflammatory responses, e.g. in the context of cancer therapy, but may also provide new therapeutic targets to counteract chronic inflammation in the context of various chronic inflammatory disorders.
Keywords: Autoimmunity; Cytokines; FcαRI; Inflammation; Myeloid cells.
Conflict of interest statement
D.L.P.B. is an employee of UCB.
Figures
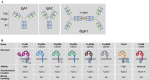
Fig. 1
The human Fc receptor family. a IgA molecules consist of two domains, which are linked by a hinge region. IgA2 molecules have a shorter hinge region than IgA1, leading to a more Y-shaped conformation. The antigen-binding domain (Fab) binds to antigens, while the crystallizable fragment (Fc) domain can be recognized by Fc receptors. Furthermore, one molecule is made up of two identical heavy chains (in blue) and two identical light chains (in green). IgA molecules can be expressed as dimers when the Fc domains are connected to each other by a joining (J) chain. b Human FcRs are divided according to their binding capability to antibody subtype, FcγR, FcεR, and FcαR. FcγRs can be further subdivided into three types: FcγRI, FcγRII, and FcγRIII, which can be grouped based on their binding affinity to IgG (with FcγRI being the only high-affinity receptor). FcαRI is genetically located on a distinct location apart from the other receptors. The human FcR family differs quite significantly from the mouse FcR family
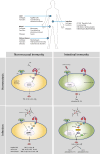
Fig. 2
Tissue-specific FcαRI-mediated control of cytokine production in homeostasis and infection. FcαRI mediates both inflammatory and immunosuppressive responses in a cell-type and tissue-specific manner. FcαRI directs cytokine production under different conditions. Homeostasis: in non-mucosal tissues, monomeric IgA recognition by FcαRI during homeostasis leads to inhibition of pro-inflammatory cytokine production through ITAMi-mediated SHP-1 recruitment. In intestinal immunity, CD103+ DCs tolerogenic conditioning leads to activation through PRRs, which results into induction of regulatory T cells and IL-10 production. Infection: bacterial infection IgA immune complex formation is recognized by FcαRI leading to cross-talk with PRRs and pro-inflammatory cytokine production in Kupffer cells, monocytes, and macrophages through Syk and PI3K-mediated up-regulation of transcription resulting in distinct expression of cytokines. Upon intestinal infection, IgA opsonization provides a second signal through FcαRI activating Syk, PI3K, and TBK1-IKKε, which increases the glycolytic flux and fatty acid synthesis (FAS) that results in an inflammatory response mediated by increased mRNA translation and caspase-1 activation
Similar articles
- The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease.
Aleyd E, Heineke MH, van Egmond M. Aleyd E, et al. Immunol Rev. 2015 Nov;268(1):123-38. doi: 10.1111/imr.12337. Immunol Rev. 2015. PMID: 26497517 Review. - Serum IgA Immune Complexes Promote Proinflammatory Cytokine Production by Human Macrophages, Monocytes, and Kupffer Cells through FcαRI-TLR Cross-Talk.
Hansen IS, Hoepel W, Zaat SAJ, Baeten DLP, den Dunnen J. Hansen IS, et al. J Immunol. 2017 Dec 15;199(12):4124-4131. doi: 10.4049/jimmunol.1700883. Epub 2017 Nov 8. J Immunol. 2017. PMID: 29118246 - IgA Fc receptors.
Monteiro RC, Van De Winkel JG. Monteiro RC, et al. Annu Rev Immunol. 2003;21:177-204. doi: 10.1146/annurev.immunol.21.120601.141011. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12524384 Review. - Anti-inflammatory role of the IgA Fc receptor (CD89): from autoimmunity to therapeutic perspectives.
Ben Mkaddem S, Rossato E, Heming N, Monteiro RC. Ben Mkaddem S, et al. Autoimmun Rev. 2013 Apr;12(6):666-9. doi: 10.1016/j.autrev.2012.10.011. Epub 2012 Nov 29. Autoimmun Rev. 2013. PMID: 23201915 Review. - The Fc receptor for IgA (FcalphaRI, CD89).
Otten MA, van Egmond M. Otten MA, et al. Immunol Lett. 2004 Mar 29;92(1-2):23-31. doi: 10.1016/j.imlet.2003.11.018. Immunol Lett. 2004. PMID: 15081523 Review.
Cited by
- Effect of zearalenone on the jejunum of weaned gilts through the Epac1/Rap1/JNK pathway.
Liu H, Ma L, Fu J, Ma X, Gao Y, Xie Y, Yuan X, Wang Y, Yang W, Jiang S. Liu H, et al. J Anim Sci. 2024 Jan 3;102:skae208. doi: 10.1093/jas/skae208. J Anim Sci. 2024. PMID: 39051732 - N-Glycosylation and Inflammation; the Not-So-Sweet Relation.
Radovani B, Gudelj I. Radovani B, et al. Front Immunol. 2022 Jun 27;13:893365. doi: 10.3389/fimmu.2022.893365. eCollection 2022. Front Immunol. 2022. PMID: 35833138 Free PMC article. Review. - IgA Complexes Induce Neutrophil Extracellular Trap Formation More Potently Than IgG Complexes.
Gimpel AK, Maccataio A, Unterweger H, Sokolova MV, Schett G, Steffen U. Gimpel AK, et al. Front Immunol. 2022 Jan 13;12:761816. doi: 10.3389/fimmu.2021.761816. eCollection 2021. Front Immunol. 2022. PMID: 35095840 Free PMC article. - Genomic landscape of patients with germline RUNX1 variants and familial platelet disorder with myeloid malignancy.
Yu K, Deuitch N, Merguerian M, Cunningham L, Davis J, Bresciani E, Diemer J, Andrews E, Young A, Donovan F, Sood R, Craft K, Chong S, Chandrasekharappa S, Mullikin J, Liu PP. Yu K, et al. Blood Adv. 2024 Jan 23;8(2):497-511. doi: 10.1182/bloodadvances.2023011165. Blood Adv. 2024. PMID: 38019014 Free PMC article. - Effect of Tourist Activities on Fecal and Salivary Glucocorticoids and Immunoglobulin A in Female Captive Asian Elephants in Thailand.
Kosaruk W, Brown JL, Plangsangmas T, Towiboon P, Punyapornwithaya V, Silva-Fletcher A, Thitaram C, Khonmee J, Edwards KL, Somgird C. Kosaruk W, et al. Animals (Basel). 2020 Oct 21;10(10):1928. doi: 10.3390/ani10101928. Animals (Basel). 2020. PMID: 33096598 Free PMC article.
References
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Career Development grant/Maag Lever Darm Stichting
- AMC fellowship 2015/Academisch Medisch Centrum
- VICI/Nederlandse Organisatie voor Wetenschappelijk Onderzoek
- Consolidator grant/European Research Council/International
LinkOut - more resources
Full Text Sources
Miscellaneous