Response to Fungal Dysbiosis by Gut-Resident CX3CR1+ Mononuclear Phagocytes Aggravates Allergic Airway Disease - PubMed (original) (raw)
Comment
. 2018 Dec 12;24(6):847-856.e4.
doi: 10.1016/j.chom.2018.11.003. Epub 2018 Nov 29.
Irina Leonardi 1, Alexa Semon 1, Itai Doron 1, Iris H Gao 2, Gregory Garbès Putzel 3, Youngjun Kim 4, Hiroki Kabata 3, David Artis 5, William D Fiers 1, Amanda E Ramer-Tait 6, Iliyan D Iliev 7
Affiliations
- PMID: 30503509
- PMCID: PMC6292739
- DOI: 10.1016/j.chom.2018.11.003
Comment
Response to Fungal Dysbiosis by Gut-Resident CX3CR1+ Mononuclear Phagocytes Aggravates Allergic Airway Disease
Xin Li et al. Cell Host Microbe. 2018.
Abstract
Sensing of the gut microbiota, including fungi, regulates mucosal immunity. Whether fungal sensing in the gut can influence immunity at other body sites is unknown. Here we show that fluconazole-induced gut fungal dysbiosis has persistent effects on allergic airway disease in a house dust mite challenge model. Mice with a defined community of bacteria, but lacking intestinal fungi were not susceptible to fluconazole-induced dysbiosis, while colonization with a fungal mixture recapitulated the detrimental effects. Gut-resident mononuclear phagocytes (MNPs) expressing the fractalkine receptor CX3CR1 were essential for the effect of gut fungal dysbiosis on peripheral immunity. Depletion of CX3CR1+ MNPs or selective inhibition of Syk signaling downstream of fungal sensing in these cells ameliorated lung allergy. These results indicate that disruption of intestinal fungal communities can have persistent effects on peripheral immunity and aggravate disease severity through fungal sensing by gut-resident CX3CR1+ MNPs.
Keywords: CX3CR1(+) mononuclear phagocytes; fungi; gut-lung axis; mycobiome; mycobiota dysbiosis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
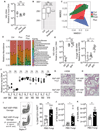
Figure 1.. Fluconazole-induced gut fungal dysbiosis has persistent immune effects on lung allergic airway disease.
(A) Layout of the experiment setup, all mice from normal drinking water (Ctrl), fluconazole (Fluc) and post-fluconazole treated groups (Post-Fluc) were intranasally immunized with HDM. (B) Blood samples were collected for serum fluconazole assessment by liquid chromatography-mass spectrometry (LC-MS/MS). Ctrl (n=8), Fluc (n=8). (C-E) All mice (n=5 per group) were intranasally immunized with HDM. Representative eosinophil staining (pre-gated on CD45+CD11c− cells; left) and frequencies of Siglec-F+SSChi eosinophils in BAL (right). (D) Left to right: total cells, eosinophil, macrophage and neutrophil numbers in BAL. (E) Frequencies of CD4+ IL-4+ Th2 cells (left) and IL-17+ Th17 cells (right) in the medLN. (F-G) ELISA detection of total serum IgE and anti-HDM IgG1. OD value relative to control group. Ctrl (n=10), Fluc (n=5), Post-Fluc (n=10). (H-I) Feces were collected from mice in A) before (Ctrl, n=10), during (Fluc, n=10), and 3 weeks after removal (Post-Fluc, n=10) of fluconazole. (H) Quantitative real-time PCR (q-PCR) for fungal 18S rDNA in feces. (n=5 per group). (I) Alpha diversity (Observed OTU index) and (J) NMDS plot (Bray-Curtis) derived from ITS amplicon sequencing data. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA.
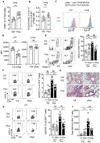
Figure 2.. Antifungal treatment does not exacerbate allergic airway disease in mycobiome-free (MyF) mice while oral supplementation with fungi is detrimental.
(A) Q-PCR for bacterial 16S rDNA in feces from mice in Figure 1H. (B) Alpha diversity (Observed OTU index) by 16S rDNA sequencing. (C) NMDS plot (Bray-Curtis) for bacterial OTUs in feces. (D) Relative bacterial abundance at the family level (n=5 per group). (E) Q-PCR for 16S rDNA (left) and 18S rDNA (right) in feces from germ-free (GF) mice (n=5), MyF-ASF mice (n=8) and WT SPF mice (n=6). (F) Species specific q-PCR for bacterial species of altered Schaedler flora in feces from MyF-ASF mice administrated normal water (n=4) or fluconazole for 3 weeks (n=5). (G) MyF-ASF mice were administrated water (Ctrl, n=4) or fluconazole water (Fluc, n=4) 7 days prior to HDM immunizations. H&E stained lung sections (20X), scale bar= 100 μM. (H-K) Mice were fed either with PBS (n=7) or a mixture of three fungal species (A. amstelodami, E. nigrum and W. sebi; n=7) for a week prior to exposure to HDM. (H) H&E stained lung histology (20X), scale bar= 100 μM. (I) Representative eosinophil staining (left), frequencies (right), (J) number of eosinophils and (K) total number of cells in BAL. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of at least two or three independent experiments. *p < 0.05, **p < 0.01, Mann-Whitney test.
Figure 3.. The systemic effects of gut fungal dysbiosis on lung allergy are mediated by CX3CR1+ MNPs.
(A) _Cd11c-Cre_−/− Cx3cr1 DTR littermates (Litt) (n=14) and Cd11c-Cre+/− Cx3cr1 DTR (ΔCX3CR1) (n=12) mice were treated with DT as described in Figure S4A. CD11b+CX3CR1+ MNPs were gated within live CD45+MHC+II+CD11c+ cells (left) and CX3CR1+MNP frequencies of in the colonic lamina propria (cLP) and lung are shown (right). (B-F) Litt and ΔCX3CR1 mice were administrated normal water (open bars, Litt (n=6), ΔCX3CR1 (n=6)) or fluconazole water (filled black bars, Litt (n=7), ΔCX3CR1 (n=6)) and immunized with HDM (Figure S4D). (B-C) Representative eosinophil staining (B, left), frequencies (B, right) and (C) total number of cells in BAL. (D) medLN total cell numbers. (E) Representative staining (left) and frequencies (right) of CD4+ GATA3+Th2 cells in the lung. (F) Frequencies of CD4+IL-4+ Th2 cells in medLN. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of two independent experiments. *p < 0.05, **p < 0.01, Mann-Whitney test.
Figure 4.. Syk signaling in intestinal CX3CR1+ MNPs contributes to allergic airway inflammation during gut fungal dysbiosis.
(A-B) Mice were treated following experimental setup outlined in Figure 1A, Ctrl (n=6), Fluc (n=8). Frequencies of CD4+ T cells (A) and CX3CR1+MNPs (B) in the lung are shown. (C-D) Littermates (Litt, n=8) and Syk fl/fl Cx3cr1-Cre-ERT mice (ΔSyk, n=7) were treated (i.p) with 4-OHT as described in Figure S4F. (C) Representative Syk staining of CX3CR1+ MNPs and (D) mean fluorescence intensity (MFI) in intestinal cLP and lung. (E-I) Litt and ΔSyk mice were administrated normal water (open bars, Litt (n=5), ΔSyk (n=8)) or fluconazole water (filled black bars, Litt (n=8), ΔCX3CR (n=7)) and immunized with HDM. (E) Representative plots and frequencies of eosinophils in BAL, (F) Representative plots (left) and frequencies (right) of CD4+ GATA3+ Th2 cells in the lung. (G) H&E stained lung sections. (20X), scale bar= 100 μM. (H-I) Representative plots (H, left), frequencies (H, right) and total number of CD4+ GATA3+ Th2 cells (I) in the cLP. Dots represent individual mice, data are represented as mean ± SEM. Data are representative of two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 Mann-Whitney test.
Comment on
- Exploring the Gut Fungi-Lung Allergy Axis.
Shibuya A, Shibuya K. Shibuya A, et al. Cell Host Microbe. 2018 Dec 12;24(6):755-757. doi: 10.1016/j.chom.2018.11.012. Cell Host Microbe. 2018. PMID: 30543774
Similar articles
- CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi.
Leonardi I, Li X, Semon A, Li D, Doron I, Putzel G, Bar A, Prieto D, Rescigno M, McGovern DPB, Pla J, Iliev ID. Leonardi I, et al. Science. 2018 Jan 12;359(6372):232-236. doi: 10.1126/science.aao1503. Science. 2018. PMID: 29326275 Free PMC article. - Exploring the Gut Fungi-Lung Allergy Axis.
Shibuya A, Shibuya K. Shibuya A, et al. Cell Host Microbe. 2018 Dec 12;24(6):755-757. doi: 10.1016/j.chom.2018.11.012. Cell Host Microbe. 2018. PMID: 30543774 - Intestinal Fungal Dysbiosis Is Associated With Visceral Hypersensitivity in Patients With Irritable Bowel Syndrome and Rats.
Botschuijver S, Roeselers G, Levin E, Jonkers DM, Welting O, Heinsbroek SEM, de Weerd HH, Boekhout T, Fornai M, Masclee AA, Schuren FHJ, de Jonge WJ, Seppen J, van den Wijngaard RM. Botschuijver S, et al. Gastroenterology. 2017 Oct;153(4):1026-1039. doi: 10.1053/j.gastro.2017.06.004. Epub 2017 Jun 15. Gastroenterology. 2017. PMID: 28624575 - The possible mechanisms of the human microbiome in allergic diseases.
Ipci K, Altıntoprak N, Muluk NB, Senturk M, Cingi C. Ipci K, et al. Eur Arch Otorhinolaryngol. 2017 Feb;274(2):617-626. doi: 10.1007/s00405-016-4058-6. Epub 2016 Apr 26. Eur Arch Otorhinolaryngol. 2017. PMID: 27115907 Review. - Fungal dysbiosis: immunity and interactions at mucosal barriers.
Iliev ID, Leonardi I. Iliev ID, et al. Nat Rev Immunol. 2017 Oct;17(10):635-646. doi: 10.1038/nri.2017.55. Epub 2017 Jun 12. Nat Rev Immunol. 2017. PMID: 28604735 Free PMC article. Review.
Cited by
- Traditional Chinese medicine to improve immune imbalance of asthma: focus on the adjustment of gut microbiota.
Lu K, Li C, Men J, Xu B, Chen Y, Yan P, Gai Z, Zhang Q, Zhang L. Lu K, et al. Front Microbiol. 2024 Oct 1;15:1409128. doi: 10.3389/fmicb.2024.1409128. eCollection 2024. Front Microbiol. 2024. PMID: 39411430 Free PMC article. Review. - The gut-lung axis: the impact of the gut mycobiome on pulmonary diseases and infections.
Sey EA, Warris A. Sey EA, et al. Oxf Open Immunol. 2024 Jul 24;5(1):iqae008. doi: 10.1093/oxfimm/iqae008. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39193472 Free PMC article. Review. - Gut Mycobiome and Asthma.
Kanj AN, Skalski JH. Kanj AN, et al. J Fungi (Basel). 2024 Mar 1;10(3):192. doi: 10.3390/jof10030192. J Fungi (Basel). 2024. PMID: 38535201 Free PMC article. Review. - Fungal microbiota sustains lasting immune activation of neutrophils and their progenitors in severe COVID-19.
Kusakabe T, Lin WY, Cheong JG, Singh G, Ravishankar A, Yeung ST, Mesko M, DeCelie MB, Carriche G, Zhao Z, Rand S, Doron I, Putzel GG, Worgall S, Cushing M, Westblade L, Inghirami G, Parkhurst CN, Guo CJ, Schotsaert M, García-Sastre A, Josefowicz SZ, Salvatore M, Iliev ID. Kusakabe T, et al. Nat Immunol. 2023 Nov;24(11):1879-1889. doi: 10.1038/s41590-023-01637-4. Epub 2023 Oct 23. Nat Immunol. 2023. PMID: 37872315 Free PMC article. - Systemic Candidiasis in Mice: New Insights From an Old Model.
Jungnickel B, Jacobsen ID. Jungnickel B, et al. Front Fungal Biol. 2022 Jun 23;3:940884. doi: 10.3389/ffunb.2022.940884. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746206 Free PMC article. Review.
References
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7, 307ra152. - PubMed
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, and White TC (2012). Hidden killers: human fungal infections. Sci Transl Med 4, 165rv113. - PubMed
- Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, Reynolds LA, Hacker L, Mohr J, Finlay BB, et al. (2017). Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous