Targeted Delivery of Bioactive Molecules for Vascular Intervention and Tissue Engineering - PubMed (original) (raw)
Review
Targeted Delivery of Bioactive Molecules for Vascular Intervention and Tissue Engineering
Hannah A Strobel et al. Front Pharmacol. 2018.
Abstract
Cardiovascular diseases are the leading cause of death in the United States. Treatment often requires surgical interventions to re-open occluded vessels, bypass severe occlusions, or stabilize aneurysms. Despite the short-term success of such interventions, many ultimately fail due to thrombosis or restenosis (following stent placement), or incomplete healing (such as after aneurysm coil placement). Bioactive molecules capable of modulating host tissue responses and preventing these complications have been identified, but systemic delivery is often harmful or ineffective. This review discusses the use of localized bioactive molecule delivery methods to enhance the long-term success of vascular interventions, such as drug-eluting stents and aneurysm coils, as well as nanoparticles for targeted molecule delivery. Vascular grafts in particular have poor patency in small diameter, high flow applications, such as coronary artery bypass grafting (CABG). Grafts fabricated from a variety of approaches may benefit from bioactive molecule incorporation to improve patency. Tissue engineering is an especially promising approach for vascular graft fabrication that may be conducive to incorporation of drugs or growth factors. Overall, localized and targeted delivery of bioactive molecules has shown promise for improving the outcomes of vascular interventions, with technologies such as drug-eluting stents showing excellent clinical success. However, many targeted vascular drug delivery systems have yet to reach the clinic. There is still a need to better optimize bioactive molecule release kinetics and identify synergistic biomolecule combinations before the clinical impact of these technologies can be realized.
Keywords: aneurysm; drug delivery; drug eluting stent; nanoparticle; vascular graft; vascular repair; vascular tissue engineering.
Figures
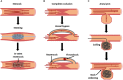
FIGURE 1
Current interventions for vascular diseases, and their modes of failure. Stenosis (A), caused by atherosclerosis or intimal hyperplasia, is frequently treated with stent placement [blue balloon in (A) used to deploy stent] to restore patency. However, in-stent restenosis is a frequent complication. In severe cases of occlusion (B), a complete vessel bypass may be necessary. With bypass grafting, there is a risk of failure due to thrombosis or restenosis at graft anastomoses. Aneurysms (C) can be treated with an aneurysm coil, to fill the aneurysmal sac and prevent further dilation. However, over time coils can begin to leak, allowing fluid to re-enter and further enlarge the aneurysm.
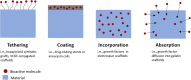
FIGURE 2
Methods for incorporating bioactive molecules into medical devices or tissue engineered grafts. Growth factors can be tethered to graft surfaces, incorporated directly into material coatings, incorporated directly within scaffold materials during fabrication, or absorbed into the material post-fabrication.
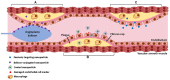
FIGURE 3
Mechanisms of nanoparticle delivery. Nanoparticles can be delivered directly to the lesion site by balloon angioplasty (A), they can be targeted using conjugated antibodies that target specific surface markers on the lesion (B), or they can passively diffuse into the lesion due to increased endothelial permeability (C).
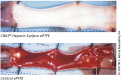
FIGURE 4
Effect of heparin on synthetic graft thrombosis. A GORE ePTFE vascular graft with or without a CBAS heparin surface coating, following a 2-h implantation in a canine carotid model. Thrombosis is clearly visible in the graft without the CBAS heparin surface. Figure reprinted from (Biran and Pond, 2017) with permission from W. L. Gore and Associates.
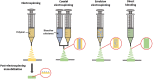
FIGURE 5
Techniques for incorporating bioactive molecules within electrospun grafts. Molecules can be tethered to the material after electrospinning. With coaxial electrospinning, the material and bioactive substance are combined during the electrospinning process, while being dispensed from two separate syringes. Alternatively, with emulsion electrospinning or direct blending a solution of bioactive molecules is mixed with the material prior to electrospinning.
Similar articles
- Clinical Effectiveness and Cost Effectiveness of Intracoronary Brachytherapy and Drug Eluting Stents [Internet].
Mørland B, Kløw NE, Rotevatn S, Steigen T, Vatne K, Wisløff T, Kristiansen IS, Norderhaug I. Mørland B, et al. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2004. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 08-2004. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2004. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 08-2004. PMID: 29320006 Free Books & Documents. Review. - Fenestrated endovascular grafts for the repair of juxtarenal aortic aneurysms: an evidence-based analysis.
Medical Advisory Secretariat. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2009;9(4):1-51. Epub 2009 Jul 1. Ont Health Technol Assess Ser. 2009. PMID: 23074534 Free PMC article. - Intravascular brachytherapy for peripheral vascular disease.
Andras A, Hansrani M, Stewart M, Stansby G. Andras A, et al. Cochrane Database Syst Rev. 2014 Jan 8;2014(1):CD003504. doi: 10.1002/14651858.CD003504.pub2. Cochrane Database Syst Rev. 2014. PMID: 24399686 Free PMC article. Review. - Recent progress in percutaneous coronary intervention: evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stent.
Doostzadeh J, Clark LN, Bezenek S, Pierson W, Sood PR, Sudhir K. Doostzadeh J, et al. Coron Artery Dis. 2010 Jan;21(1):46-56. doi: 10.1097/MCA.0b013e328333f550. Coron Artery Dis. 2010. PMID: 19952925 Review. - Endovascular stent grafting and open surgical replacement for chronic thoracic aortic aneurysms: a systematic review and prospective cohort study.
Sharples L, Sastry P, Freeman C, Gray J, McCarthy A, Chiu YD, Bicknell C, McMeekin P, Vallabhaneni SR, Cook A, Vale L, Large S. Sharples L, et al. Health Technol Assess. 2022 Jan;26(6):1-166. doi: 10.3310/ABUT7744. Health Technol Assess. 2022. PMID: 35094747
Cited by
- Accelerated and Improved Vascular Maturity after Transplantation of Testicular Tissue in Hydrogels Supplemented with VEGF- and PDGF-Loaded Nanoparticles.
Del Vento F, Poels J, Vermeulen M, Ucakar B, Giudice MG, Kanbar M, des Rieux A, Wyns C. Del Vento F, et al. Int J Mol Sci. 2021 May 28;22(11):5779. doi: 10.3390/ijms22115779. Int J Mol Sci. 2021. PMID: 34071329 Free PMC article. - Advancements in textile techniques for cardiovascular tissue replacement and repair.
Bakare A, Mohanadas HP, Tucker N, Ahmed W, Manikandan A, Faudzi AAM, Mohamaddan S, Jaganathan SK. Bakare A, et al. APL Bioeng. 2024 Oct 17;8(4):041503. doi: 10.1063/5.0231856. eCollection 2024 Dec. APL Bioeng. 2024. PMID: 39431050 Free PMC article. Review. - A Comparative Study of an Anti-Thrombotic Small-Diameter Vascular Graft with Commercially Available e-PTFE Graft in a Porcine Carotid Model.
Lee KS, Kayumov M, Emechebe GA, Kim DW, Cho HJ, Jeong YJ, Lee DW, Park JK, Park CH, Kim CS, Obiweluozor FO, Jeong IS. Lee KS, et al. Tissue Eng Regen Med. 2022 Jun;19(3):537-551. doi: 10.1007/s13770-021-00422-4. Epub 2022 Feb 15. Tissue Eng Regen Med. 2022. PMID: 35167044 Free PMC article. - Enhanced hemocompatibility and rapid magnetic anastomosis of electrospun small-diameter artificial vascular grafts.
Liu P, Liu X, Yang L, Qian Y, Lu Q, Shi A, Wei S, Zhang X, Lv Y, Xiang J. Liu P, et al. Front Bioeng Biotechnol. 2024 Jan 24;12:1331078. doi: 10.3389/fbioe.2024.1331078. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38328445 Free PMC article. - A head-to-head comparison of conjugation methods for VHHs: Random maleimide-thiol coupling versus controlled click chemistry.
van Moorsel MVA, Urbanus RT, Verhoef S, Koekman CA, Vink M, Vermonden T, Maas C, Pasterkamp G, Schiffelers RM. van Moorsel MVA, et al. Int J Pharm X. 2019 Jun 21;1:100020. doi: 10.1016/j.ijpx.2019.100020. eCollection 2019 Dec. Int J Pharm X. 2019. PMID: 31517285 Free PMC article.
References
- Abizaid A., Costa R. A., Schofer J., Ormiston J., Maeng M., Witzenbichler B., et al. (2016). Serial multimodality imaging and 2-year clinical outcomes of the novel DESolve novolimus-eluting bioresorbable coronary scaffold system for the treatment of single de novo coronary lesions. JACC Cardiovasc. Interv. 9 565–574. 10.1016/j.jcin.2015.12.004 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources