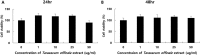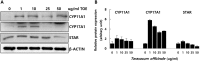Steroidogenic effects of Taraxacum officinale extract on the levels of steroidogenic enzymes in mouse Leydig cells - PubMed (original) (raw)
Steroidogenic effects of Taraxacum officinale extract on the levels of steroidogenic enzymes in mouse Leydig cells
Hyun Joo Chung et al. Anim Cells Syst (Seoul). 2018.
Abstract
In this study, we investigated the steroidogenic effect of Taraxacum officinale extract on mouse TM3 Leydig cells, which produce male hormones by increasing the levels of steroidogenic enzymes. Steroidogenic enzymes are involved in the production of testosterone in the testis. To date, the steroidogenic effect of T. officinale has not been reported. Therefore, we examined the steroidogenic effects of T. officinale extract (TOE) on mouse Leydig cells in vitro. Traditionally, plants have been used for the treatment of various kinds of ailments. For many years, some medicinal plants have been used to regulate steroidogenesis or late-onset hypogonadism (LOH). In particular, plants belonging to the genus Taraxacum have anti-inflammatory, anti-nociceptive, anti-oxidant, and anti-cancer properties. In this study, we determined whether the TOE exerts steroidogenic effects by increasing the levels of enzymes associated with steroidogenesis, such as the steroidogenic acute regulatory protein (STAR), CYP11A1, and translocator protein (TSPO) in the mitochondria and CYP17A1 in the smooth endoplasmic reticulum, in mouse Leydig cells. Our results showed that the TOE significantly increased the mRNA and protein levels of steroidogenic enzymes, thereby increasing the testosterone levels in mouse Leydig cells. Thus, our results indicate that the TOE increases the levels of steroidogenic enzymes, and further studies are required to establish the potential of this plant in regulating steroidogenesis and improving LOH.
Keywords: Leydig cell; Taraxacum officinale extract (TOE); late-onset hypogonadism (LOH); medicinal plant; steroidogenesis.
Figures
Figure 1.
HPLC chromatograms of standard sample (A) and Taraxacum officinale extract (B). Peak1, Chicoric acid. The compound in T. officinale was identified on the basis of the retention time and chromatogram pattern, by co-chromatography with an authentic standard. From the data presented, peak 1 with a retention time of 18.45 min, was chicoric acid. The contents of chicoric acid in our study had high level of around 2.6 mg/g.
Figure 2.
Cell viability of TM3 Leydig cells treated Taraxacum officinale extract. The varying concentration of 0, 1, 10, 25 and 50 µg/ml of T. officinale were treated into TM3 cells for 24(A) and 48 h(B). The experiments were performed in triplicate.
Figure 3.
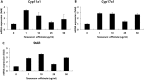
Measurement of mRNA levels of Cyp11a1, Cyp17a1 and StAR in mouse Leydig cells. Cells were treated with 0, 1, 10, 25 and 50 µg/ml of T. officinale for 24 h. The qRT-PCR was performed with the gene-specific primers for Cyp11a1 (A), Cyp17a1 (B) and StAR (C). mRNA were normalized with 18s rRNA gene. The experiments were performed in triplicate.
Figure 4.
Measurement of protein levels of CYP11A1, CYP17A1 and STAR in mouse TM3 Leydig cells. Cells were treated with 0, 1, 10, 25 and 50 µg/ml of T. officinale for 48 h. The Western blotting analysis was performed with the gene-specific antibodies for CYP11A1, CYP17A1 and STAR (A). Total protein were normalized with ß-ACTIN. The data were quantified by using Image J program (B). The experiments were performed in triplicate.
Figure 5.
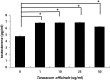
Measurement of levels of testosterone in mouse Leydig cell supernatants. Cells were treated with 0, 1, 10, 25 and 50 µg/ml of T. officinale for 48 h. The cell supernatant were collected and used to do the ELISA method. Each group's _p_-value was calculated. (*p < .05, **p < .001). The experiments were performed in three times, respectively.
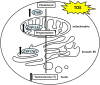
Figure 6.
The model of steroidogenesis with the treatment of TOE in mouse Leydig cell.
Similar articles
- Korean Ginseng Berry Extract Enhances the Male Steroidogenesis Enzymes In Vitro and In Vivo.
Chung HJ, Lee SJ, Jang A, Lee CE, Lee DW, Myung SC, Kim JW. Chung HJ, et al. World J Mens Health. 2023 Apr;41(2):446-459. doi: 10.5534/wjmh.220075. Epub 2023 Jan 2. World J Mens Health. 2023. PMID: 36649918 Free PMC article. - [Xiongcan Yishen Prescription up-regulates the expressions of cholesterol transport proteins, steroidogenic enzymes and SF-1 in the Leydig cells of rats with late-onset hypogonadism].
Zhou X, Li BN, Zhou HL, He QH, Zhou Q, Xiong W, Gao RS, Lin QF, Shang XJ. Zhou X, et al. Zhonghua Nan Ke Xue. 2020 Mar;26(3):258-264. Zhonghua Nan Ke Xue. 2020. PMID: 33346967 Chinese. - The SET protein promotes androgen production in testicular Leydig cells.
Zhang B, Ma W, Zhu Q, Xu W, Gao L, Xu B, Xu S, Gao C, Gao L, Liu J, Cui Y. Zhang B, et al. Andrology. 2018 May;6(3):478-487. doi: 10.1111/andr.12476. Epub 2018 Feb 26. Andrology. 2018. PMID: 29481720 Free PMC article. - Leydig cell aging and hypogonadism.
Beattie MC, Adekola L, Papadopoulos V, Chen H, Zirkin BR. Beattie MC, et al. Exp Gerontol. 2015 Aug;68:87-91. doi: 10.1016/j.exger.2015.02.014. Epub 2015 Feb 18. Exp Gerontol. 2015. PMID: 25700847 Free PMC article. Review. - Leydig cells: formation, function, and regulation.
Zirkin BR, Papadopoulos V. Zirkin BR, et al. Biol Reprod. 2018 Jul 1;99(1):101-111. doi: 10.1093/biolre/ioy059. Biol Reprod. 2018. PMID: 29566165 Free PMC article. Review.
Cited by
- Phytochemicals: Alternative for Infertility Treatment and Associated Conditions.
Chorosho SH, Malik N, Panesar G, Kumari P, Jangra S, Kaur R, Al-Ghamdi MS, Albishi TS, Chopra H, Singh R, Murthy HCA. Chorosho SH, et al. Oxid Med Cell Longev. 2023 May 11;2023:1327562. doi: 10.1155/2023/1327562. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 37215366 Free PMC article. Review. - The seahorse (Hippocampus comes L.) extract ameliorates sperm qualities, testosterone level, and serum biochemistry in rats induced by depo medroxyprogesterone acetate.
Mundijo T, Suyatna FD, Wibowo AE, Lestari SW, Yusra Y, Midoen YH. Mundijo T, et al. J Adv Vet Anim Res. 2023 Mar 31;10(1):126-131. doi: 10.5455/javar.2023.j661. eCollection 2023 Mar. J Adv Vet Anim Res. 2023. PMID: 37155542 Free PMC article. - Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure.
Pfingstgraf IO, Taulescu M, Pop RM, Orăsan R, Vlase L, Uifalean A, Todea D, Alexescu T, Toma C, Pârvu AE. Pfingstgraf IO, et al. Antioxidants (Basel). 2021 Mar 24;10(4):504. doi: 10.3390/antiox10040504. Antioxidants (Basel). 2021. PMID: 33804908 Free PMC article. - Apoptosis, inflammation, and oxidative stress in infertility: A mini review.
Ojo OA, Nwafor-Ezeh PI, Rotimi DE, Iyobhebhe M, Ogunlakin AD, Ojo AB. Ojo OA, et al. Toxicol Rep. 2023 Apr 13;10:448-462. doi: 10.1016/j.toxrep.2023.04.006. eCollection 2023. Toxicol Rep. 2023. PMID: 37125147 Free PMC article. Review. - Screening and Identification of Differential Ovarian Proteins before and after Induced Ovulation via Seminal Plasma in Bactrian Camels.
Wang Q, Zhang Q, Li Y, Zhao X, Zhang Y. Wang Q, et al. Animals (Basel). 2021 Dec 9;11(12):3512. doi: 10.3390/ani11123512. Animals (Basel). 2021. PMID: 34944287 Free PMC article.
References
- Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR BR.. 2002. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 23:439–447. - PubMed
Grants and funding
This work was supported by a grant [714001-07] from the Center for Industrialization of Natural Nutraceuticals through the Agriculture, Food and Rural Affairs Research Center Support Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea. Also this research was supported by the Chung-Ang University Research Scholarship Grants in 2017.
LinkOut - more resources
Full Text Sources