Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine - PubMed (original) (raw)
Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine
Els van Westen et al. Vaccines (Basel). 2018.
Abstract
The two currently available ten- and thirteen-valent pneumococcal conjugate vaccines (PCV10 and PCV13) both induce serotype-specific IgG anti-polysaccharide antibodies and are effective in preventing vaccine serotype induced invasive pneumococcal disease (IPD) as well as in reducing overall vaccine-serotype carriage and transmission and thereby inducing herd protection in the whole population. IgG levels decline after vaccination and could become too low to prevent carriage acquisition and/or pneumococcal disease. We compared the levels of 10-valent (PCV10) and 13-valent (PCV13) pneumococcal vaccine induced serum IgG antibodies at multiple time points after primary vaccinations. Data from two separate studies both performed in the Netherlands in infants vaccinated at 2, 3, and 4 months of age with either PCV10 or PCV13 were compared. Antibody levels were measured at 5, 8, and 11 months of age, during the interval between the primary immunization series and the 11-months booster dose. Serotype-specific IgG levels were determined by multiplex immunoassay. Although antibody kinetics showed significant variation between serotypes and between vaccines for the majority of the 10 shared serotypes, i.e., 1, 5, 7F, 9V, 14, 18C, and 23F, antibody concentrations were sufficiently high for both vaccines, immediately after the primary series and throughout the whole period until the booster dose. In contrast, for serotypes 4 and 19F in the PCV10 group and for serotypes 4 and 6B in the PCV13 group, IgG antibody concentrations already come within reach of the frequently used seroprotection level of 0.35 μg/mL immediately after the primary series at the five month time point and/or at eight months. This paper addresses the importance of revealing differences in serotype-specific and pneumococcal vaccine-dependent IgG antibody patterns during the interval between the primary series and the booster dose, an age period with a high IPD incidence. Trial registration: www.trialregister.nl NTR3069 and NTR2316.
Keywords: IgG; PCV10; PCV13; antibody kinetics; pneumococcal conjugate vaccine; serotype-specific.
Conflict of interest statement
E.A.M.S. declares to have received unrestricted research support from Pfizer, grant support for vaccine studies from Pfizer and GlaxoSmithKline plc, and fees paid to the institution for advisory boards or participation in independent data monitoring committees for Pfizer and GSK. None of these fees or grants have been received for the research described in this paper. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Figure 1
Study design and disposition of subjects in clinical trials NTR2316 and NTR3069.
Figure 2
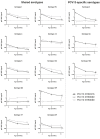
Serotype-specific IgG concentrations at 5, 8, and 11 months of age. IgG levels were expressed as geometric mean concentrations (GMCs) with 95% CIs of infants vaccinated with PCV10 or PCV13. Age at blood sampling was set as a continuous variable. For serotype 6A, the GMC was 0.06 μg/mL at 5 months in the PCV10 group.
Similar articles
- Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: a randomised controlled trial.
Temple B, Toan NT, Dai VTT, Bright K, Licciardi PV, Marimla RA, Nguyen CD, Uyen DY, Balloch A, Huu TN, Mulholland EK. Temple B, et al. Lancet Infect Dis. 2019 May;19(5):497-509. doi: 10.1016/S1473-3099(18)30734-5. Epub 2019 Apr 8. Lancet Infect Dis. 2019. PMID: 30975525 Free PMC article. Clinical Trial. - Waning of antibody levels induced by a 13-valent pneumococcal conjugate vaccine, using a 3 + 0 schedule, within the first year of life among children younger than 5 years in Blantyre, Malawi: an observational, population-level, serosurveillance study.
Swarthout TD, Henrion MYR, Thindwa D, Meiring JE, Mbewe M, Kalizang'Oma A, Brown C, Msefula J, Moyo B, Mataya AA, Barnaba S, Pearce E, Gordon M, Goldblatt D, French N, Heyderman RS. Swarthout TD, et al. Lancet Infect Dis. 2022 Dec;22(12):1737-1747. doi: 10.1016/S1473-3099(22)00438-8. Epub 2022 Aug 24. Lancet Infect Dis. 2022. PMID: 36029796 Free PMC article. - Differential B-cell memory around the 11-month booster in children vaccinated with a 10- or 13-valent pneumococcal conjugate vaccine.
van Westen E, Wijmenga-Monsuur AJ, van Dijken HH, van Gaans-van den Brink JA, Kuipers B, Knol MJ, Berbers GA, Sanders EA, Rots NY, van Els CA. van Westen E, et al. Clin Infect Dis. 2015 Aug 1;61(3):342-9. doi: 10.1093/cid/civ274. Epub 2015 Apr 1. Clin Infect Dis. 2015. PMID: 25838290 Free PMC article. Clinical Trial. - The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on serotype 19A invasive pneumococcal disease.
Principi N, Esposito S. Principi N, et al. Expert Rev Vaccines. 2015;14(10):1359-66. doi: 10.1586/14760584.2015.1075884. Epub 2015 Aug 7. Expert Rev Vaccines. 2015. PMID: 26289973 Review. - Pneumococcal serotype evolution in Western Europe.
Tin Tin Htar M, Christopoulou D, Schmitt HJ. Tin Tin Htar M, et al. BMC Infect Dis. 2015 Oct 14;15:419. doi: 10.1186/s12879-015-1147-x. BMC Infect Dis. 2015. PMID: 26468008 Free PMC article. Review.
Cited by
- Hospitalisation outcomes in pneumococcal-vaccinated versus -unvaccinated patients with exacerbation of COPD: results from the HOPE COPD Study.
Venkitakrishnan R, Vijay A, Augustine J, Ramachandran D, Cleetus M, Nirmal AS, John S. Venkitakrishnan R, et al. ERJ Open Res. 2023 May 2;9(3):00476-2022. doi: 10.1183/23120541.00476-2022. eCollection 2023 Jul. ERJ Open Res. 2023. PMID: 37143841 Free PMC article. - Pneumococcal Vaccines: Challenges and Prospects.
Licciardi P, Papadatou I. Licciardi P, et al. Vaccines (Basel). 2019 Feb 27;7(1):25. doi: 10.3390/vaccines7010025. Vaccines (Basel). 2019. PMID: 30818791 Free PMC article. - Caveolae-Mediated Extracellular Vesicle (CMEV) Signaling of Polyvalent Polysaccharide Vaccination: A Host-Pathogen Interface Hypothesis.
Li SC, Kabeer MH. Li SC, et al. Pharmaceutics. 2022 Nov 30;14(12):2653. doi: 10.3390/pharmaceutics14122653. Pharmaceutics. 2022. PMID: 36559147 Free PMC article. Review. - Immunogenicity of Current and Next-Generation Pneumococcal Conjugate Vaccines in Children: Current Challenges and Upcoming Opportunities.
Feemster K, Buchwald UK, Banniettis N, Joyce JG, Velentgas P, Chapman TJ, Yildirim I. Feemster K, et al. Open Forum Infect Dis. 2024 May 6;11(5):ofae220. doi: 10.1093/ofid/ofae220. eCollection 2024 May. Open Forum Infect Dis. 2024. PMID: 38770212 Free PMC article. Review. - No Waning of Pneumococcal Vaccine Responses over Time in People with Inflammatory Arthritis: Findings from a Single Centre Cohort.
Nagra D, Bechman K, Russell MD, Yang Z, Adas M, Subesinghe S, Rutherford A, Alveyn E, Patel S, Wincup C, Mahto A, Baldwin C, Karafotias I, Cope A, Norton S, Galloway J. Nagra D, et al. Vaccines (Basel). 2024 Jan 10;12(1):69. doi: 10.3390/vaccines12010069. Vaccines (Basel). 2024. PMID: 38250882 Free PMC article.
References
- Van Deursen A.M., van Mens S.P., Sanders E.A., Vlaminckx B.J., de Melker H.E., Schouls L.M., de Greeff S.C., van der Ende A., the Invasive Pneumococcal Disease Sentinel Surveillance Laboratory Group Invasive pneumococcal disease and 7-valent pneumococcal conjugate vaccine, The Netherlands. Emerg. Infect. Dis. 2012;18:1729–1737. doi: 10.3201/eid1811.120329. - DOI - PMC - PubMed
- Elberse K.E., van der Heide H.G., Witteveen S., van de Pol I., Schot C.S., van der Ende A., Berbers G.A., Schouls L.M. Changes in the composition of the pneumococcal population and in IPD incidence in The Netherlands after the implementation of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2012;30:7644–7651. doi: 10.1016/j.vaccine.2012.04.021. - DOI - PubMed
- Spijkerman J., van Gils E.J., Veenhoven R.H., Hak E., Yzerman E.P., van der Ende A., Wijmenga-Monsuur A.J., van den Dobbelsteen G.P., Sanders E.A. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, The Netherlands. Emerg. Infect. Dis. 2011;17:584–591. doi: 10.3201/eid1704.101115. - DOI - PMC - PubMed
- Dagan R., Patterson S., Juergens C., Greenberg D., Givon-Lavi N., Porat N., Gurtman A., Gruber W.C., Scott D.A. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: A randomized double-blind trial. Clin. Infect. Dis. 2013;57:952–962. doi: 10.1093/cid/cit428. - DOI - PubMed
- Ben-Shimol S., Greenberg D., Givon-Lavi N., Schlesinger Y., Somekh E., Aviner S., Miron D., Dagan R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: An active prospective nationwide surveillance. Vaccine. 2014;32:3452–3459. doi: 10.1016/j.vaccine.2014.03.065. - DOI - PubMed
LinkOut - more resources
Full Text Sources