Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity - PubMed (original) (raw)
Review
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity
Amy Proal et al. Front Pediatr. 2018.
Abstract
The illness ME/CFS has been repeatedly tied to infectious agents such as Epstein Barr Virus. Expanding research on the human microbiome now allows ME/CFS-associated pathogens to be studied as interacting members of human microbiome communities. Humans harbor these vast ecosystems of bacteria, viruses and fungi in nearly all tissue and blood. Most well-studied inflammatory conditions are tied to dysbiosis or imbalance of the human microbiome. While gut microbiome dysbiosis has been identified in ME/CFS, microbes and viruses outside the gut can also contribute to the illness. Pathobionts, and their associated proteins/metabolites, often control human metabolism and gene expression in a manner that pushes the body toward a state of illness. Intracellular pathogens, including many associated with ME/CFS, drive microbiome dysbiosis by directly interfering with human transcription, translation, and DNA repair processes. Molecular mimicry between host and pathogen proteins/metabolites further complicates this interference. Other human pathogens disable mitochondria or dysregulate host nervous system signaling. Antibodies and/or clonal T cells identified in patients with ME/CFS are likely activated in response to these persistent microbiome pathogens. Different human pathogens have evolved similar survival mechanisms to disable the host immune response and host metabolic pathways. The metabolic dysfunction driven by these organisms can result in similar clusters of inflammatory symptoms. ME/CFS may be driven by this pathogen-induced dysfunction, with the nature of dysbiosis and symptom presentation varying based on a patient's unique infectious and environmental history. Under such conditions, patients would benefit from treatments that support the human immune system in an effort to reverse the infectious disease process.
Keywords: ME/CFS; autoantibody; dysbiosis; gut-brain axis; holobiont; microbiome; molecular mimicry; pathobiont.
Figures
Figure 1
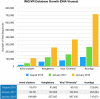
The IMG/VR database catalogs viruses in Earth's ecosystems including the human body. Viral diversity in IMG/VR has more than tripled since August 2016.
Figure 2
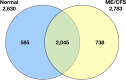
Venn diagram of the qualitative distribution of proteins identified in cerebrospinal fluid from normal control subjects and ME/CFS subjects. Seven hundred and thirty eight of 2,783 identified proteins (26.5%) were unique to patients with ME/CFS. The numbers of proteins for each category separately is shown outside the circles (2,630 for normal controls, 2,783 for ME/CFS) (94).
Figure 3
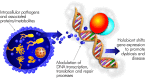
Intracellular pathogens and the proteins/metabolites they express can directly interfere with human transcription, translation, and DNA repair processes.
Figure 4
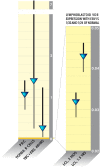
VDR activity is downregulated as much as 20 times in Epstein Barr Virus-infected lymphoblastoid cell lines (128).
Similar articles
- The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS).
König RS, Albrich WC, Kahlert CR, Bahr LS, Löber U, Vernazza P, Scheibenbogen C, Forslund SK. König RS, et al. Front Immunol. 2022 Jan 3;12:628741. doi: 10.3389/fimmu.2021.628741. eCollection 2021. Front Immunol. 2022. PMID: 35046929 Free PMC article. Review. - Microbe-microbe and host-microbe interactions drive microbiome dysbiosis and inflammatory processes.
Proal AD, Lindseth IA, Marshall TG. Proal AD, et al. Discov Med. 2017 Jan;23(124):51-60. Discov Med. 2017. PMID: 28245427 Review. - Identification of CD8 T-cell dysfunction associated with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID and treatment with a nebulized antioxidant/anti-pathogen agent in a retrospective case series.
Gil A, Hoag GE, Salerno JP, Hornig M, Klimas N, Selin LK. Gil A, et al. Brain Behav Immun Health. 2023 Dec 27;36:100720. doi: 10.1016/j.bbih.2023.100720. eCollection 2024 Mar. Brain Behav Immun Health. 2023. PMID: 38327880 Free PMC article. - A systematic review of enteric dysbiosis in chronic fatigue syndrome/myalgic encephalomyelitis.
Du Preez S, Corbitt M, Cabanas H, Eaton N, Staines D, Marshall-Gradisnik S. Du Preez S, et al. Syst Rev. 2018 Dec 20;7(1):241. doi: 10.1186/s13643-018-0909-0. Syst Rev. 2018. PMID: 30572962 Free PMC article. - Does the microbiome and virome contribute to myalgic encephalomyelitis/chronic fatigue syndrome?
Newberry F, Hsieh SY, Wileman T, Carding SR. Newberry F, et al. Clin Sci (Lond). 2018 Mar 9;132(5):523-542. doi: 10.1042/CS20171330. Print 2018 Mar 15. Clin Sci (Lond). 2018. PMID: 29523751 Free PMC article. Review.
Cited by
- Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers.
Hajjo R, Sabbah DA, Al Bawab AQ. Hajjo R, et al. Diagnostics (Basel). 2022 Jul 19;12(7):1742. doi: 10.3390/diagnostics12071742. Diagnostics (Basel). 2022. PMID: 35885645 Free PMC article. Review. - Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis.
Sfera A, Osorio C, Zapata Martín Del Campo CM, Pereida S, Maurer S, Maldonado JC, Kozlakidis Z. Sfera A, et al. Front Cell Neurosci. 2021 Jun 25;15:673217. doi: 10.3389/fncel.2021.673217. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34248502 Free PMC article. - More than 50 long-term effects of COVID-19: a systematic review and meta-analysis.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. Lopez-Leon S, et al. Sci Rep. 2021 Aug 9;11(1):16144. doi: 10.1038/s41598-021-95565-8. Sci Rep. 2021. PMID: 34373540 Free PMC article. - Is It Useful to Question the Recovery Behaviour of Patients with ME/CFS or Long COVID?
Vink M, Vink-Niese F. Vink M, et al. Healthcare (Basel). 2022 Feb 18;10(2):392. doi: 10.3390/healthcare10020392. Healthcare (Basel). 2022. PMID: 35207003 Free PMC article. Review.
References
- Chapenko S, Krumina A, Logina I, Rasa S, Chistjakovs M, Sultanova M, et al. . Association of active human herpesvirus-6,−7 and parvovirus b19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv Virol. (2012) 2012:205085. 10.1155/2012/205085 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources