Contemporaneous radiations of fungi and plants linked to symbiosis - PubMed (original) (raw)
doi: 10.1038/s41467-018-07849-9.
Michael D Nowak 2, Michael E Alfaro 3, Valérie Reeb 4, Jolanta Miadlikowska 1, Michael Krug 5, A Elizabeth Arnold 6 7, Louise A Lewis 8, David L Swofford 1 9, David Hibbett 10, Khidir Hilu 11, Timothy Y James 12, Dietmar Quandt 5, Susana Magallón 13
Affiliations
- PMID: 30575731
- PMCID: PMC6303338
- DOI: 10.1038/s41467-018-07849-9
Contemporaneous radiations of fungi and plants linked to symbiosis
François Lutzoni et al. Nat Commun. 2018.
Abstract
Interactions between fungi and plants, including parasitism, mutualism, and saprotrophy, have been invoked as key to their respective macroevolutionary success. Here we evaluate the origins of plant-fungal symbioses and saprotrophy using a time-calibrated phylogenetic framework that reveals linked and drastic shifts in diversification rates of each kingdom. Fungal colonization of land was associated with at least two origins of terrestrial green algae and preceded embryophytes (as evidenced by losses of fungal flagellum, ca. 720 Ma), likely facilitating terrestriality through endomycorrhizal and possibly endophytic symbioses. The largest radiation of fungi (Leotiomyceta), the origin of arbuscular mycorrhizae, and the diversification of extant embryophytes occurred ca. 480 Ma. This was followed by the origin of extant lichens. Saprotrophic mushrooms diversified in the Late Paleozoic as forests of seed plants started to dominate the landscape. The subsequent diversification and explosive radiation of Agaricomycetes, and eventually of ectomycorrhizal mushrooms, were associated with the evolution of Pinaceae in the Mesozoic, and establishment of angiosperm-dominated biomes in the Cretaceous.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
Contemporaneous changes revealed from independent estimates of divergence times for plants (top) and fungi (bottom) aligned using a single time scale. For clarity, only the most relevant error bars are shown for each divergence time estimate (see Supplementary Figs. 2, 3 for error bars). Exceptional shifts in diversification rates, highlighted by a change in color on the branches, are numbered on each tree (within yellow circles) and placed before the node where a shift was detected. The yellow circles numbered “1” indicate the basal rate relative to which the first major accelerations or decelerations were detected. Numbers at the tips of the trees refer to monophyletic groups listed to the left of each tree. Each taxon name listed on the left side is followed by its known species richness in parentheses. The asterisk after prasinophyceans indicates that this is a paraphyletic group of nine lineages of green algae currently classified at the rank of class or order, or as clades without formal taxonomic description. Three wide beige vertical bars spanning both trees highlight major events in the evolutionary history of fungi and plants that were defined by the origin of fungal key innovations, including traits that enabled mutualistic symbioses with plants. Two light green vertical bars delimit time periods when plant key innovations, such as seeds, enabled plants to form inland forests, and angiosperm diversification rates drastically accelerated, profoundly affecting the evolution of fungi. The light red vertical bar spanning both trees highlights a period when the most important diversification of plants (during the early evolution of tracheophytes) and the largest radiation of fungi (the Leotiomyceta radiation; yellow circle number 5) took place, starting around 450 Ma. Each arc represents an important event in the biology of plants (green arcs) and fungi (black arcs), including putative origins of major plant–fungal symbioses (arbuscular mycorrhizal [AM], endophytic and endolichenic [endophytes], ascolichenic [ascolichens], and ectomycorrhizal [ECM]). Horizontal dashed lines with arrows indicate major transitions from aquatic to terrestrial environments or from terrestrial wet to terrestrial dry (inland) forests, or the expansion of fungal lignin-degrading peroxidases
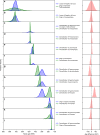
Fig. 2
Pairwise comparison of selected land plant and fungal divergence times (node ages) posterior densities and their differences. a–l Positive age differences indicate that the fungal evolutionary event preceded the plant evolutionary event; negative age differences indicate that the plant evolutionary event preceded the fungal event
Similar articles
- A phylogenetic estimation of trophic transition networks for ascomycetous fungi: are lichens cradles of symbiotrophic fungal diversification?
Arnold AE, Miadlikowska J, Higgins KL, Sarvate SD, Gugger P, Way A, Hofstetter V, Kauff F, Lutzoni F. Arnold AE, et al. Syst Biol. 2009 Jun;58(3):283-97. doi: 10.1093/sysbio/syp001. Epub 2009 Jul 11. Syst Biol. 2009. PMID: 20525584 - Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi.
Sánchez-García M, Ryberg M, Khan FK, Varga T, Nagy LG, Hibbett DS. Sánchez-García M, et al. Proc Natl Acad Sci U S A. 2020 Dec 22;117(51):32528-32534. doi: 10.1073/pnas.1922539117. Epub 2020 Nov 30. Proc Natl Acad Sci U S A. 2020. PMID: 33257574 Free PMC article. - Broad compatibility in fungal root symbioses.
Zuccaro A, Lahrmann U, Langen G. Zuccaro A, et al. Curr Opin Plant Biol. 2014 Aug;20:135-45. doi: 10.1016/j.pbi.2014.05.013. Epub 2014 Jun 13. Curr Opin Plant Biol. 2014. PMID: 24929298 Review. - Symbiotic options for the conquest of land.
Field KJ, Pressel S, Duckett JG, Rimington WR, Bidartondo MI. Field KJ, et al. Trends Ecol Evol. 2015 Aug;30(8):477-86. doi: 10.1016/j.tree.2015.05.007. Epub 2015 Jun 22. Trends Ecol Evol. 2015. PMID: 26111583 Review.
Cited by
- Flowering plant immune repertoires expand under mycorrhizal symbiosis.
Kramer EM, Statter SA, Yi HJ, Carlson JW, McClelland DHR. Kramer EM, et al. Plant Direct. 2019 Mar 5;3(3):e00125. doi: 10.1002/pld3.125. eCollection 2019 Mar. Plant Direct. 2019. PMID: 31245768 Free PMC article. - The taxonomy of the model filamentous fungus Podospora anserina.
Ament-Velásquez SL, Johannesson H, Giraud T, Debuchy R, Saupe SJ, Debets AJM, Bastiaans E, Malagnac F, Grognet P, Peraza-Reyes L, Gladieux P, Kruys Å, Silar P, Huhndorf SM, Miller AN, Vogan AA. Ament-Velásquez SL, et al. MycoKeys. 2020 Nov 25;75:51-69. doi: 10.3897/mycokeys.75.55968. eCollection 2020. MycoKeys. 2020. PMID: 33281477 Free PMC article. - Genes related to redox and cell curvature facilitate interactions between Caulobacter strains and Arabidopsis.
Berrios L, Ely B. Berrios L, et al. PLoS One. 2021 Apr 1;16(4):e0249227. doi: 10.1371/journal.pone.0249227. eCollection 2021. PLoS One. 2021. PMID: 33793620 Free PMC article. - Sulfur assimilation using gaseous carbonyl sulfide by the soil fungus Trichoderma harzianum.
Iizuka R, Hattori S, Kosaka Y, Masaki Y, Kawano Y, Ohtsu I, Hibbett D, Katayama Y, Yoshida M. Iizuka R, et al. Appl Environ Microbiol. 2024 Feb 21;90(2):e0201523. doi: 10.1128/aem.02015-23. Epub 2024 Feb 1. Appl Environ Microbiol. 2024. PMID: 38299812 Free PMC article. - Chronic Ionizing Radiation of Plants: An Evolutionary Factor from Direct Damage to Non-Target Effects.
Duarte GT, Volkova PY, Fiengo Perez F, Horemans N. Duarte GT, et al. Plants (Basel). 2023 Mar 4;12(5):1178. doi: 10.3390/plants12051178. Plants (Basel). 2023. PMID: 36904038 Free PMC article. Review.
References
- Kirk, P. M., Cannon, P. F., Minter, D. W. & Stalpers, J. A. Dictionary of the Fungi (CAB International, Wallingford, 2008).
- Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis 3rd edn (Academic Press, Boston, 2008).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical