Transfusion of red blood cells stored for shorter versus longer duration for all conditions - PubMed (original) (raw)
Transfusion of red blood cells stored for shorter versus longer duration for all conditions
Akshay Shah et al. Cochrane Database Syst Rev. 2018.
Abstract
Background: Red blood cell (RBC) transfusion is a common treatment for anaemia in many conditions. The safety and efficacy of transfusing RBC units that have been stored for different durations before a transfusion is a current concern. The duration of storage for a RBC unit can be up to 42 days. If evidence from randomised controlled trials (RCT) were to indicate that clinical outcomes are affected by storage duration, the implications for inventory management and clinical practice would be significant.
Objectives: To assess the effects of using red blood cells (RBCs) stored for a shorter versus a longer duration, or versus RBCs stored for standard practice duration, in people requiring a RBC transfusion.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, PubMed (for epublications), LILACS, Transfusion Evidence Library, Web of Science CPCI-S and four international clinical trial registries on 20 November 2017.
Selection criteria: We included RCTs that compared transfusion of RBCs of shorter versus longer storage duration, or versus standard practice storage duration.
Data collection and analysis: We used standard Cochrane methods.
Main results: We included 22 trials (42,835 participants) in this review.The GRADE quality of evidence ranged from very low to moderate for our primary outcome of in-hospital and short-term mortality reported at different time points.Transfusion of RBCs of shorter versus longer storage duration Eleven trials (2249 participants) compared transfusion of RBCs of shorter versus longer storage duration. Two trials enrolled low birth weight neonates, two enrolled children with severe anaemia secondary to malaria or sickle cell disease, and eight enrolled adults across a range of clinical settings (intensive care, cardiac surgery, major elective surgery, hospitalised in-patients, haematology outpatients). We judged only two trials to be at low risk of bias across all domains; most trials had an unclear risk for multiple domains.Transfusion of RBCs of shorter versus longer storage duration probably leads to little or no difference in mortality at seven-day follow-up (risk ratio (RR) 1.42, 95% confidence interval (CI) 0.66 to 3.06; 1 trial, 3098 participants; moderate quality evidence) or 30-day follow-up (RR 0.85, 95%CI 0.50 to 1.45; 2 trials, 1121 participants; moderate quality evidence) in adults undergoing major elective cardiac or non-cardiac surgery.For neonates, no studies reported on the primary outcome of in-hospital or short-term mortality. At 40 weeks gestational age, the effect of RBCs of shorter versus longer storage duration on the risk of death was uncertain, as the quality of evidence is very low (RR 0.90, 95% CI 0.41 to 1.85; 1 trial, 52 participants).The effect of RBCs of shorter versus longer storage duration on the risk of death in children with severe anaemia was also uncertain within 24 hours of transfusion (RR 1.50, 95% CI 0.43 to 5.25; 2 trials, 364 participants; very low quality evidence), or at 30-day follow-up (RR 1.40, 95% CI 0.45 to 4.31; 1 trial, 290 participants; low quality evidence).Only one trial, in children with severe anaemia (290 participants), reported adverse transfusion reactions. Only one child in each arm experienced an adverse reaction within 24 hours of transfusion.Transfusion of RBCs of shorter versus standard practice storage duration Eleven trials (40,588 participants) compared transfusion of RBCs of shorter versus standard practice storage duration. Three trials enrolled critically ill term neonates; two of these enrolled very low birth weight neonates. There were no trials in children. Eight trials enrolled critically ill and non-critically ill adults, with most being hospitalised. We judged four trials to be at low risk of bias across all domains with the others having an unclear risk of bias across multiple domains.Transfusion of RBCs of shorter versus standard practice storage duration probably leads to little or no difference in adult in-hospital mortality (RR 1.05, 95% CI 0.97 to 1.14; 4 trials, 25,704 participants; moderate quality evidence), ICU mortality (RR 1.06, 95% CI 0.98 to 1.15; 3 trials, 13,066 participants; moderate quality evidence), or 30-day mortality (RR 1.04, 95% CI 0.96 to 1.13; 4 trials, 7510 participants;moderate quality evidence).Two of the three trials that enrolled neonates reported that there were no adverse transfusion reactions. One trial reported an isolated case of cytomegalovirus infection in participants assigned to the standard practice storage duration group. Two trials in critically ill adults reported data on transfusion reactions: one observed no difference in acute transfusion reactions between arms (RR 0.67, 95% CI 0.19 to 2.36, 2413 participants), but the other observed more febrile nonhaemolytic reactions in the shorter storage duration arm (RR 1.48, 95% CI 1.13 to 1.95, 4919 participants).Trial sequential analysis showed that we may now have sufficient evidence to reject a 5% relative risk increase or decrease of death within 30 days when transfusing RBCs of shorter versus longer storage duration across all patient groups.
Authors' conclusions: The effect of storage duration on clinically important outcomes has now been investigated in large, high quality RCTs, predominantly in adults. There appears to be no evidence of an effect on mortality that is related to length of storage of transfused RBCs. However, the quality of evidence in neonates and children is low. The current practice in blood banks of using the oldest available RBCs can be continued safely. Additional RCTs are not required, but research using alternative study designs, should focus on particular subgroups (e.g. those requiring multiple RBC units) and on factors affecting RBC quality.
Conflict of interest statement
Akshay Shah: This report is independent research supported by the National Institute for Health Research (NIHR Doctoral Research Fellowship, Dr Akshay Shah, DRF‐2017‐10‐094). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Susan Brunskill: none known
Michael Desborough: none known
Carolyn Dorée: none known
Marialena Trivella: I was a Training Co‐ordinator at the UK Cochrane Centre and am a statistical editor or peer reviewer for five Cochrane groups (Anaesthesia, Wounds, Injuries, Breast Cancer and Sexually Transmitted Infections). My work with the Cochrane groups is independent of my involvement in this review. My involvement in this review did not involve a financial relationship.
Simon Stanworth: none known
Figures
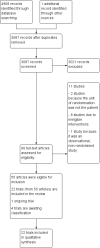
1
A study flow diagram
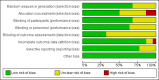
2
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Twenty‐two studies are included in this review.
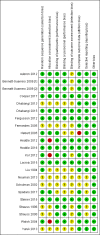
3
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Update of
- Transfusion of fresher versus older red blood cells for all conditions.
Brunskill SJ, Wilkinson KL, Doree C, Trivella M, Stanworth S. Brunskill SJ, et al. Cochrane Database Syst Rev. 2015 May 12;(5):CD010801. doi: 10.1002/14651858.CD010801.pub2. Cochrane Database Syst Rev. 2015. PMID: 25963030 Updated. Review.
Similar articles
- Red blood cell transfusion management for people undergoing cardiac surgery for congenital heart disease.
Wilkinson KL, Kimber C, Allana A, Dorée C, Champaneria R, Brunskill SJ, Murphy MF. Wilkinson KL, et al. Cochrane Database Syst Rev. 2025 Mar 19;3(3):CD009752. doi: 10.1002/14651858.CD009752.pub3. Cochrane Database Syst Rev. 2025. PMID: 40105353 Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Transfusion of fresher versus older red blood cells for all conditions.
Brunskill SJ, Wilkinson KL, Doree C, Trivella M, Stanworth S. Brunskill SJ, et al. Cochrane Database Syst Rev. 2015 May 12;(5):CD010801. doi: 10.1002/14651858.CD010801.pub2. Cochrane Database Syst Rev. 2015. PMID: 25963030 Updated. Review. - Erythropoietin plus iron versus control treatment including placebo or iron for preoperative anaemic adults undergoing non-cardiac surgery.
Kaufner L, von Heymann C, Henkelmann A, Pace NL, Weibel S, Kranke P, Meerpohl JJ, Gill R. Kaufner L, et al. Cochrane Database Syst Rev. 2020 Aug 13;8(8):CD012451. doi: 10.1002/14651858.CD012451.pub2. Cochrane Database Syst Rev. 2020. PMID: 32790892 Free PMC article. - Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support.
Radford M, Estcourt LJ, Sirotich E, Pitre T, Britto J, Watson M, Brunskill SJ, Fergusson DA, Dorée C, Arnold DM. Radford M, et al. Cochrane Database Syst Rev. 2024 May 23;5(5):CD011305. doi: 10.1002/14651858.CD011305.pub3. Cochrane Database Syst Rev. 2024. PMID: 38780066 Free PMC article. Review.
Cited by
- Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells.
Möller MN, Orrico F, Villar SF, López AC, Silva N, Donzé M, Thomson L, Denicola A. Möller MN, et al. ACS Omega. 2022 Dec 28;8(1):147-168. doi: 10.1021/acsomega.2c06768. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643550 Free PMC article. Review. - Age of Red Cells for Transfusion and Outcomes in Patients with ARDS.
Graw JA, Bünger V, Materne LA, Krannich A, Balzer F, Francis RCE, Pruß A, Spies CD, Kuebler WM, Weber-Carstens S, Menk M, Hunsicker O. Graw JA, et al. J Clin Med. 2022 Jan 4;11(1):245. doi: 10.3390/jcm11010245. J Clin Med. 2022. PMID: 35011986 Free PMC article. - The oxygen dissociation curve of blood in COVID-19-An update.
Böning D, Kuebler WM, Vogel D, Bloch W. Böning D, et al. Front Med (Lausanne). 2023 Feb 27;10:1098547. doi: 10.3389/fmed.2023.1098547. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36923010 Free PMC article. Review. - Colloid osmotic pressure of contemporary and novel transfusion products.
Klanderman RB, Bosboom JJ, Korsten H, Zeiler T, Musson REA, Veelo DP, Geerts BF, van Bruggen R, de Korte D, Vlaar APJ. Klanderman RB, et al. Vox Sang. 2020 Nov;115(8):664-675. doi: 10.1111/vox.12932. Epub 2020 May 6. Vox Sang. 2020. PMID: 32378239 Free PMC article. - Red blood cell transfusion management for people undergoing cardiac surgery for congenital heart disease.
Wilkinson KL, Kimber C, Allana A, Dorée C, Champaneria R, Brunskill SJ, Murphy MF. Wilkinson KL, et al. Cochrane Database Syst Rev. 2025 Mar 19;3(3):CD009752. doi: 10.1002/14651858.CD009752.pub3. Cochrane Database Syst Rev. 2025. PMID: 40105353 Review.
References
References to studies included in this review
Aubron 2012 {published data only}
- Aubron C, Syres G, Nichol A, Bailey M, Board J, Magrin G, et al. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion 2012;52:1196‐202. - PubMed
Bennett‐Guerrero 2009 [1] {published data only}
- Bennett‐Guerrero E, Stafford‐Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, et al. A prospective, double blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion 2009;49:1375‐83. - PubMed
Bennett‐Guerrero 2009 [2] {published data only}
- Bennett‐Guerrero E, Stafford‐Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, et al. A prospective, double blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion 2009;49:1375‐83. - PubMed
Cooper 2017 {published data only}
- Cooper DJ, McQuilten ZK, Nichol A, Ady B, Aubron C, Bailey M, et al. Age of red cells for transfusion and outcomes in critically ill adults. New England Journal of Medicine 2017;377:1858‐67. - PubMed
- Kaukonen KM, Cooper DJ. Age of blood and mortality in critically ill patients: the TRANSFUSE trial. Intensive Care Medicine 2013;39:S386.
Dhabangi 2013 {published data only}
Dhabangi 2015 {published and unpublished data}
- Dhabangi A, Ainomugisha B, Cserti‐Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia. The TOTAL randomized clinical trial. JAMA 2015;314(23):2514‐23. - PubMed
- NCT01586923. RBC Transfusion in Severe Anemia With Lactic Acidosis (TOTAL). www.clinicaltrials.gov.
- Shah A. Clarification of the time‐points of the additional deaths beyond the study's initial 24‐ hour observation period. Email to: W Dzik 10 January 2018.
Fergusson 2012 {published data only}
- Fergusson D, Hutton B, Hogan DL, LeBel L, Blajchman MA, Ford JC, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfusion Medicine Reviews 2009;23(1):55‐61. - PubMed
- Fergusson DA. Assessing the clinical effect of RBCs in neonates: the age of red blood cells in premature infants (ARIPI) trial. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology 2012;40:203‐38.
- Fergusson DA. The age of red blood cells in neonates ‐ The ARIPI trial. Transfusion Alternatives in Transfusion Medicine 2012;12(2):5.
- Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez‐Bouali N, Smyth JA, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low‐birth‐weight infants: the ARIPI randomized trial. JAMA 2012;308(14):1443‐51. - PubMed
- Fergusson DA, Hebert PC, LeBel L, Rouvinez‐Bouali NG, Smyth JA, Sankaran K, et al. Age of red blood cells in premature infants (ARIPI). Transfusion 2012;52:11A‐12A. - PubMed
Fernandes 2005 {published data only}
- Cunha DH, Kopelman BI, Santos AM, Guinsburg R, Terzian CN, Kuwano ST, et al. Can red blood cells stored up to 30 days be safely transfused to very low birthweight infants?. Pediatric Research 2004;55(68):2622.
- Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, Areco KN, Guinsburg R, Araujo Peres C, et al. Transfusions of CPDA‐1 red blood cells stored for up to 28 days decrease donor exposures in very low‐birth‐weight premature infants. Transfusion Medicine 2005; Vol. 15:467‐73. - PubMed
Hebert 2005 {published data only}
- Hebert PC, Chin‐Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesthesia and Analgesia 2005;100:1433‐8. - PubMed
Heddle 2012 {published data only}
- Heddle NM, Cook RJ, Arnold DM, Crowther MA, Warkentin TE, Webert KE, et al. The effect of blood storage duration on in‐hospital mortality: a randomized controlled pilot feasibility trial. Transfusion 2012;52:1203‐12. - PubMed
- Heddle NM, Eikelboom JW, Cook RJ, Liu Y, Barty RL, Warkentin TE, et al. Can we do a 25,000 patient pragmatic study to address the age of blood and mortality controversy? A pilot to determine feasibility. Transfusion 2011;51:193A.
Heddle 2016 {published data only}
- Cook RJ, Heddle NM, Lee KA, Arnold DM, Crowther MA, Devereaux PJ, et al. Red blood cell storage and in‐hospital mortality: a secondary analysis pf the INFORM randomised controlled trial. Lancet Haematology 2017;4(11):e544‐e552. - PubMed
- Eikelboom JW, Cook RJ, Barty R, Liu Y, Arnold DM, Crowther MA, et al. Rationale and design of the Informing Fresh versus Old Red Cell Management (INFORM) trial: an international pragmatic randomized trial. Transfusion Medicine Reviews 2016;3(1):25‐9. - PubMed
- Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of short‐term vs. long‐term blood storage of mortality after transfusion. New England Journal of Medicine 2017;375(20):1937‐45. - PubMed
- Heddle NM, Cook RJ, Barty R, Liu Y, Arnold DM, Crowther MA, et al. Informing Fresh Versus Old red cell Management (INFORM) trial: a large international pragmatic randomized trial. Transfusion. 2016; Vol. 56:5a. - PubMed
Kor 2012 {published data only}
- Kor DJ, Kashyap R, Weiskopf R, Wilson G, Buskirk CM, Winters JL, et al. The impact of red blood cell storage duration on short‐term pulmonary function and immunologic status in mechanically ventilated patients. American Journal of Respiratory and Critical Care Medicine 2011;183:1.
- NCT00751322. Transfusion‐induced alterations of pulmonary and immune function in mechanically ventilated patients (TRALI2). CliicalTrials.gov record number NCT00751322.
Lacroix 2015 {published data only}
- Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfusion Medicine Reviews 2011;25(3):197‐205. - PubMed
- Lacroix J, Hebert PC, Fergusson DA, Tinmoith A, Cook DJ, Marshall JC, et al. Length of red blood cell storage and clinical outcomes in transfused critical ill adults ‐ the Age of Blood Evaluation (ABLE) randomised controlled trial. Transfusion Medicine. 2015; Vol. 25 (Suppl 1):6. - PubMed
- Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. New England Journal of Medicine 2015;372(15):1410‐8. - PubMed
- Lehr A, Fergusson D, Sabri E, Hebert P, Tinmouth A, Lacroix J. Age of blood evaluation: a subgroup analysis or perioperative critically ill adults. Critical Care Medicine 2016;44(12 Suppl 1):460. - PubMed
- Mack JP, Kahn SR, Tinmouth A, Fergusson D, Hebert PC, Lacroix J. Dose dependent effect of stored red blood: results of a sub‐group analysis of the age of blood evaluation (ABLE) trial. Blood 2016;128:22. - PubMed
Liu 1994 {published data only}
- Liu EA, Mannino FL, Lane TA. Prospective, randomized trial of the safety and efficacy of a limited donor exposure transfusion program for premature neonates. The Journal of Pediatrics 1994;125(1):92‐6. - PubMed
Neuman 2013 {published data only}
- Neuman R, Karatela SK, Menon V, Newman JL, Polhemus D, Sher S, et al. Hospitalised patients transfused with storage aged (but not fresh) red blood cells (RBCS) demonstrate impaired endothelial function and significant reductions in circulating nitrite levels. Transfusion 2013;53(Suppl):135A.
- Neuman R, Menon V, Karatela S, Newman J, Sher S, Ashraf K, et al. Endothelial dysfunction precipitated by transfusion of storage‐aged but not fresh red blood cells. Vascular Medicine 2013;61:E2066.
- Neuman RB, Polhemus D, Karatela S. Transfusion of storage‐aged, but not fresh red blood cells in hospitalised patients results in endothelial dysfunction and decreased circulating nitrite levels. Nitric Oxide: Biology and Chemistry 2013;21:s20.
Schulman 2002 {published data only}
- Schulman CI, Nathe K, Brown M, Cohn S. Impact of age of transfused blood in the trauma patient. Journal of Trauma Injury 2002;52(6):1224‐5. - PubMed
Spadaro 2017 {published data only}
- NCT01976234. Stored RBC Transfusion and Immonomodulation. www.clinicaltrials.gov.
- Spadaro S, Taccone FS, Fogagnolo A, Fontana V, Ragazzi R, Verri M, et al. The effects of storage of red blood cells on the development of postoperative infections after noncardiac surgery. Transfusion 2017;57:2727‐37. - PubMed
Steiner 2015 {published data only}
- Steiner ME. Discussions about the RECESS trial. Email to M Murphy 30 September 2014.
Strauss 1996 {published data only}
- Strauss RG, Burmeister L, James T, Miller J, Johnson K, Bell E. A randomized trial of fresh versus stored RBCS for neonatal transfusions. Transfusion 1994;34;10(S):S269. - PubMed
- Strauss RG, Burmeister LF, Johnson K, James T, Miller J, Cordle DG, et al. AS‐1 red cells for neonatal transfusions: a randomized trial assessing donor exposure and safety. Transfusion 1996;36(10):873‐8. - PubMed
Strauss 2000 {published data only}
- Strauss RG, Burmeister L, James T, Miller J, Johnson K, Bell E. A randomized trial of fresh versus stored RBCS for neonatal transfusions. Transfusion 1994;34;10(S):S269. - PubMed
- Strauss RG, Burmeister LF, Cordle DG. Feasibility and safety of AS‐3 red blood cells stored up to 42 days for neonatal transfusions. Transfusion 1999;39(10S):S507‐0400. - PubMed
- Strauss RG, Burmeister LF, Johnson K, Cress G, Cordle D. Feasibility and safety of AS‐3 red blood cells for neonatal transfusions. Journal of Pediatrics 2000;136(2):215‐9. - PubMed
Walsh 2004 {published data only}
- Maginnis M, Maciver C, Cairns A, McArdle F, Scott N, Walsh TS, et al. Behind the scenes of the age of stored red cells randomized trial ‐ crucial role played by blood bank staff. Transfusion Medicine 2001;37(Suppl 1):P40XIX.
- Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients?. Critical Care Medicine 2004;32(2):364‐71. - PubMed
Yuruk 2013 {published data only}
- Yuruk K, Milstein MJ, Bezemer R, Barteks SA, Biemond BJ, Ince C. Transfusion of banked red blood cells and the effects on hemorrheology and microvascular hemodynamics in anemic hematology outpatients. Transfusion 2013;53:1346‐52. - PubMed
References to studies excluded from this review
Bao 2017 {published data only}
Chantepie 2015 {published data only}
- Chantepie 2015. Transfusion strategy in hematological intensive care unit. www.clinicaltrials.gov, NCT024661264. - PMC - PubMed
Eshleman 1994 {published data only}
- Eshleman JR, Akinbi H, Pleasure J, Asakura T, Magee D, Smith L, et al. Prospective double‐blind study of small volume neonatal transfusion with RBCs up to 35 days old. Transfusion 1994; Vol. 34, issue 10S:S126.
Hod 2011 {published data only}
Lebiedz 2012 {published data only}
Murphy 2017 {published data only}
- Murphy 2017. Red cell rejuvenation for the attenuation of transfusion associated organ injury in cardiac surgery. www.clinicaltrials.gov, NCT03167788.
Rapido 2017 {published data only}
Rodrigues 2015 {published data only}
- Rodrigues 2015. Strategy of transfusion in trauma patients ‐ STATA trial. www.clinicaltrials.gov, NCT02416817.
Seitelbach 2011 {published data only}
- Chia J, Seitelbach M, Chin‐Yee IH, Hsia CC. Quality of life outcomes of the age of blood in highly transfusion dependent patients with myelodysplasia: prospective randomized controlled N‐of‐1 studies. Blood 2010;116(21):4411.
- Seitelbach M, Chin‐Yee IH, Kinney J, Chia J, Ormond K, Mahon J, et al. Age of blood does not affect quality of life and hemoglobin: N‐of‐1 trials in transfusion‐dependent patients. Blood 2011;118:3369.
Wasser 1989 {published data only}
- Wasser MN, Houbiers JG, D'Amaro J, Hermans J, Huysmans HA, Konijnenburg GC, et al. The effect of fresh versus stored blood on post‐operative bleeding after coronary bypass surgery: a prospective randomized study. British Journal of Haematology 1989;72:81‐4. - PubMed
Yamal 2015 {published data only}
References to studies awaiting assessment
NCT00458783 {published data only}
- NCT00458783. Red Cell Storage Duration and Outcomes in Cardiac Surgery (The Clevelend Trial). clinicaltrials.gov/show/NCT00458783.
NCT01534676 {published data only}
- NCT01534676. Effects of Transfusion or Older Stored Red Cells. www.clinicaltrials.gov.
NCT02050230 {published data only}
- NCT02050230. Hemodynamic effects of stored blood transfusion in intensive care patients. www.clinicaltrials.gov.
NCT02724605 {published data only}
- NCT02724605. The Effect of Red Blood Cells Storage Duration on Biochemical Changes in Pediatric Patients. www.clinicaltrials.gov.
References to ongoing studies
NCT01977547 {published data only}
- NCT01977547. Age of Blood in Children in Pediatric Intensive Care Units (ABC PICU). clinicaltrials.gov/show/NCT01977547.
Additional references
Alexander 2016
- Alexander PE, Barty R, Fei Y, Vandvik PO, Pai M, Siemieniuk RA, et al. Transfusion of fresher versus older red blood cells in hospitalized patients: a systematic review and meta‐analysis. Blood 2016;127(4):400‐10. - PubMed
Alter 2008
Basran 2006
- Basran S, Frumento RJ, Cohen A, Lee S, Du Y, Nishanian E, et al. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesthesia Analgesia 2006;103:15‐20. - PubMed
Bennett‐Guerrero 2009
- Bennett‐Guerrero E, Stafford‐Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, et al. A prospective, double‐blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion 2009;49:1375‐83. - PubMed
Beutler 2006
Carson 2012
Carson 2013
- Carson JL, Carless PA, Hébert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013;309:83‐4. - PubMed
Carson 2016
Chai‐Adisaksopha 2017
- Chai‐Adisaksopha C, Alexander PE, Guyatt G, Crowther MA, Heddle NM, Devereaux PJ, et al. Mortality outcomes in patients transfused with fresher versus older red blood cells: a meta‐analysis. Vox Sanguinis 2017;112(3):268‐78. - PubMed
Cobain 2007
- Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Medicine 2007;17:1‐15. - PubMed
Corwin 2004
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: Anemia and blood transfusion in the critically ill ‐ current clinical practice in the United States. Critical Care Medicine 2004;32:39‐52. - PubMed
D'Alessandro 2010
Glynn 2010
- Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion 2010;50:1164‐9. - PubMed
Hebert 1993
- Hebert PC, Drummond AJ, Singer J, Bernard GR, Russell JA. A simple, multiple system organ failure scoring system predicts mortality of patient who have severe sepsis syndrome. Chest 1993;104:230‐5. - PubMed
Hebert 1999
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New England Journal of Medicine 1999;340:409‐17. - PubMed
Hess 2010
- Hess JR. Red cell changes during storage. Transfusion and Apheresis Science 2010;43:51‐9. - PubMed
Higgins 2011a
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Khan 2006
Koch 2008
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red‐cell storage and complications after cardiac surgery. New England Journal of Medicine 2008;358:1229‐39. - PubMed
Kucukakin 2011
- Kucukakin B, Kocak V, Lykkesfeldt J, Nielsen HJ, Magnussen K, Rosenberg J, et al. Storage‐induced increase in biomarkers of oxidative stress and inflammation in red blood cell components. Scandinavian Journal of Clinical Laboratory Investigation 2011;71:299‐303. - PubMed
Leal‐Noval 2003
- Leal‐Noval SR, Jara‐Lopez I, Garcia‐Garmendia JL, Marin‐Niebla A, Herruzo‐Aviles A, Camacho‐Larana P, et al. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology 2003;98:815‐22. - PubMed
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Lelubre 2009
- Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality?. Transfusion 2009;49:1384‐94. - PubMed
Lelubre 2013
Martin 2010
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: final data for 2008. National Vital Statistics Report 2010;59:1‐71. - PubMed
McLean 2009
- McLean E, Cogswell M, Egli I, Wojdyla D, Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993‐2005. Public Health Nutrition 2009;12:444‐54. - PubMed
Mynster 2000
- Mynster T, Nielsen HJ. The impact of storage time of transfused blood on postoperative infectious complications in rectal cancer surgery. Scandinavian Journal of Gastroenterology 2000;35:212‐7. - PubMed
NHSBT 2012
- Hospital Services, NHS Blood and Transplant, Fiton, North Bristol. Hospital Services (Issues) department data [personal communication]. Email to: Richard Gregg 15 March 2012.
NICE 2015
- National Institute for Health and Care Excellence. Blood Transfusion. NICE guideline14 2015. [www.nice.org.uk/guidance/NG24.] - PubMed
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roback 2011a
Roback 2011b
- Roback JD. American Association of Blood Banks. 17th Edition. Bethesda: American Association of Blood Banks, 2011.
Rygard 2018
- Rygard SL, Jonsson AB, Madsen MB, Perner A, Holst LB, Johansson PI, et al. Effects of shorter versus longer storage time of transfused red blood cells in adult ICU patients: a systematic review with meta‐analysis and trial sequential analysis. Intensive Care Medicine 2018;44(2):204‐17. - PubMed
Schrier 1979
- Schrier SL, Hardy B, Bensch K, Junga I, Krueger J. Red blood cell membrane storage lesion. Transfusion 1979;19:158‐65. - PubMed
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org 2011.
Sharifi 2000
- Sharifi S, Dzik WH, Sadrzadeh SM. Human plasma and tirilazad mesylate protect stored human erythrocytes against the oxidative damage of gamma‐irradiation. Transfusion Medicine 2000;10:125‐30. - PubMed
SHOT 2015
- Bolton‐Maggs PH (editor), Poles D, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2014 Annual SHOT Report. www.shotuk.org/wp‐content/uploads/myimages/report‐2014.pdf (accessed 19 December 2018).
Sohmer 1979
- Sohmer PR, Dawson RB. The significance of 2,3‐DPG in red blood cell transfusions. CRC Critical Reviews in Clinical Laboratory Sciences 1979;11:107‐74. - PubMed
Spinella 2015
- Spinella PC, Acker J. Storage duration and other measures of quality of red blood cells for transfusion. JAMA 2015;314(23):2509‐10. - PubMed
Stainsby 2006
- Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfusion Medicine Reviews 2006;20:273‐82. - PubMed
Stanger 2012
- Stanger SH, Yates N, Wilding R, Cotton S. Blood inventory management: hospital best practice. Transfusion Medicine Reviews 2012;26:153‐63. - PubMed
Stapley 2012
Steiner 2009
- Steiner ME, Stowell C. Does red blood cell storage affect clinical outcome? When in doubt, do the experiment. Transfusion 2009;49:1286‐90. - PubMed
Tettamanti 2010
TSA 2011 [Computer program]
- Copenhagen Trial Unit. Trial Sequential Analysis. Version beta 0.9. Copenhagen, Denmark: Centre for Clinical Intervention Research, Rigshospitalet, 2012.
Vamvakas 1999
- Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion 1999;39:701‐10. - PubMed
Vamvakas 2000
- Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion 2000;40:101‐9. - PubMed
Vamvakas 2010
- Vamvakas EC. Meta‐analysis of clinical studies of the purported deleterious effects of "old" (versus "fresh") red blood cells: are we at equipoise?. Transfusion 2010;50:600‐10. - PubMed
Vamvakas 2011
- Vamvakas EC. Purported deleterious effects of "old" versus "fresh" red blood cells: an updated meta‐analysis. Transfusion 2011;51(5):1122‐3. - PubMed
van de Watering 2006
- Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion 2006;46:1712‐8. - PubMed
Vincent 2002
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA 2002;288:1499‐507. - PubMed
Vora 1989
- Vora S, West C, Beutler E. The effect of additives on red cell 2,3 diphosphoglycerate levels in CPDA preservatives. Transfusion 1989;29:226‐9. - PubMed
Walsh 2010
- Walsh TS, Wyncoll DLA, Stanworth SJ. Managing anaemia in critically ill adults. BMJ 2010;341:c4408. - PubMed
Wang 2012
Wilkinson 2011
- Wilkinson KL, Brunskill SJ, Doree C, Hopewell S, Stanworth S, Murphy MF, et al. The clinical effects of red blood cell transfusions: an overview of the randomized controlled trials evidence base. Transfusion Medicine Reviews 2011;25:145‐55 e2. - PubMed
Zimrin 2009
- Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sanguinis 2009;96:93‐103. - PubMed
References to other published versions of this review
Brunskill 2015
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical