Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: A nationwide observational study - PubMed (original) (raw)
Observational Study
. 2019 May;21(5):1136-1145.
doi: 10.1111/dom.13627. Epub 2019 Feb 6.
Affiliations
- PMID: 30609272
- PMCID: PMC6593417
- DOI: 10.1111/dom.13627
Observational Study
Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: A nationwide observational study
Anna Norhammar et al. Diabetes Obes Metab. 2019 May.
Abstract
Aims: To investigate cardiovascular (CV) safety and event rates for dapagliflozin versus other glucose-lowering drugs (GLDs) in a real-world type 2 diabetes population after applying the main inclusion criteria and outcomes from the DECLARE-TIMI 58 study.
Methods: Patients with new initiation of dapagliflozin and/or other GLDs were identified in Swedish nationwide healthcare registries for the period 2013 to 2016. Patients were included if they met the main DECLARE-TIMI 58 inclusion criteria: age ≥40 years and established CV disease or presence of multiple-risk factors, e.g. men aged ≥55 years and women aged ≥60 years with hypertension or dyslipidaemia. Propensity scores for the likelihood of dapagliflozin initiation were calculated, then 1:3 matching was carried out. DECLARE-TIMI 58 outcomes were hospitalization for heart failure (HHF) or CV-specific mortality, and major adverse CV events (MACE; CV-specific mortality, myocardial infarction, or stroke). Cox survival models were used to estimate hazard ratios (HRs).
Results: After matching, a total of 28 408 new-users of dapagliflozin and/or other GLDs were identified, forming the population for the present study (henceforth referred to as the DECLARE-like cohort. The mean age of this cohort was 66 years, and 34% had established CV disease. Dapagliflozin was associated with 21% lower risk of HHF or CV mortality versus other GLDs (HR 0.79, 95% confidence interval [CI] 0.69-0.92) and had no significant association with MACE (HR 0.90, 95% CI 0.79-1.03). HHF and CV mortality risks, separately, were lower at HR 0.79 (95% CI 0.67-0.93) and HR 0.75 (95% CI 0.57-0.97), respectively. Non-significant associations were seen for myocardial infarction and stroke: HR 0.91 (95% CI 0.74-1.11) and HR 1.06 (95% CI 0.87-1.30), respectively.
Conclusion: In a real-world population similar to those included in the DECLARE-TIMI 58 study, dapagliflozin was safe with regard to CV outcomes and resulted in lower event rates of HHF and CV mortality versus other GLDs.
Keywords: cardiovascular disease; cohort study; dapagliflozin; pharmaco-epidemiology; type 2 diabetes.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
All authors participated in the research design. M.T. performed the data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and in writing the manuscript. All authors took responsibility for the decision to submit for publication.
Figures
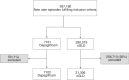
Figure 1
Flow charts for dapagliflozin versus other glucose‐lowering drug (GLD) groups. Proportions not fulfilling propensity‐matching 1:3 with 0.2 caliper were excluded and shown in grey boxes
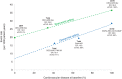
Figure 2
Correlation between baseline established cardiovascular disease (eCVD) and all‐cause mortality rates in the comparator groups from populations with multiple risk factors (MRF) and eCVD in a real‐world DECLARE‐like vs clinical trial setting. References: Neal et al NEJM 20173; Zinman et al NEJM 20154; Wiviott et al NEJM 20185, ER, event rates, events per 1000 patient‐years
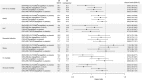
Figure 3
Forest plots comparing the real‐world DECLARE‐like results with those of other sodium‐glucose co‐transporter‐2 inhibitor cardiovascular outcome trials. The studies are presented according to mortality event rates in the comparator group, that is, highest in EMPA‐REG OUTCOME and lowest in DECLARE‐TIMI 58. References: Fitchett et al Eur Heart J 201836; Radholm et al Circulation 201837; Wiviott et al NEJM 20185. CV, cardiovascular; ER, event rate per 1000 patient‐years; HHF, hospitalization for heart failure; MACE, major adverse cardiovascular events (CV‐specific mortality, non‐fatal myocardial infarction and non‐fatal stroke)
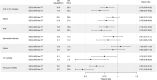
Figure 4
Forest plots comparing results from intention‐to‐treat (ITT) vs an on‐treatment (OT) analysis. CV, cardiovascular; ER, event rate per 1000 patient‐years; HHF, hospital event for heart failure; MACE, major adverse cardiovascular events (CV‐specific mortality, non‐fatal myocardial infarction and non‐fatal stroke)
Similar articles
- Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study.
Persson F, Nyström T, Jørgensen ME, Carstensen B, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Eriksson JW, Norhammar A, Bodegard J, Birkeland KI. Persson F, et al. Diabetes Obes Metab. 2018 Feb;20(2):344-351. doi: 10.1111/dom.13077. Epub 2017 Sep 8. Diabetes Obes Metab. 2018. PMID: 28771923 Free PMC article. - Dapagliflozin vs non-SGLT-2i treatment is associated with lower healthcare costs in type 2 diabetes patients similar to participants in the DECLARE-TIMI 58 trial: A nationwide observational study.
Norhammar A, Bodegard J, Nyström T, Thuresson M, Rikner K, Nathanson D, Eriksson JW. Norhammar A, et al. Diabetes Obes Metab. 2019 Dec;21(12):2651-2659. doi: 10.1111/dom.13852. Epub 2019 Aug 26. Diabetes Obes Metab. 2019. PMID: 31379124 Free PMC article. - Dapagliflozin and Cardiac, Kidney, and Limb Outcomes in Patients With and Without Peripheral Artery Disease in DECLARE-TIMI 58.
Bonaca MP, Wiviott SD, Zelniker TA, Mosenzon O, Bhatt DL, Leiter LA, McGuire DK, Goodrich EL, De Mendonca Furtado RH, Wilding JPH, Cahn A, Gause-Nilsson IAM, Johanson P, Fredriksson M, Johansson PA, Langkilde AM, Raz I, Sabatine MS. Bonaca MP, et al. Circulation. 2020 Aug 25;142(8):734-747. doi: 10.1161/CIRCULATIONAHA.119.044775. Epub 2020 Aug 3. Circulation. 2020. PMID: 32795086 Clinical Trial. - Rationale for the Early Use of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes.
Handelsman Y. Handelsman Y. Adv Ther. 2019 Oct;36(10):2567-2586. doi: 10.1007/s12325-019-01054-w. Epub 2019 Aug 23. Adv Ther. 2019. PMID: 31444707 Free PMC article. Review. - Dapagliflozin and cardiovascular outcomes in patients with Type 2 diabetes.
Al-Bazz DY, Wilding JP. Al-Bazz DY, et al. Future Cardiol. 2020 Mar;16(2):77-88. doi: 10.2217/fca-2019-0065. Epub 2020 Jan 9. Future Cardiol. 2020. PMID: 31914812 Review.
Cited by
- Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Heart Failure in Patients With Type 2 Diabetes Mellitus: A Systematic Review.
Abdelhady HA, Oumar Abakar A, Gangavarapu RR, Mahmud SA, Manandhar A, Sabir G, Malasevskaia I. Abdelhady HA, et al. Cureus. 2024 Sep 3;16(9):e68560. doi: 10.7759/cureus.68560. eCollection 2024 Sep. Cureus. 2024. PMID: 39364510 Free PMC article. Review. - Dapagliflozin treatment of patients with chronic kidney disease without diabetes across different albuminuria levels (OPTIMISE-CKD).
Svensson MK, Tangri N, Bodegård J, Adamsson Eryd S, Thuresson M, Sofue T. Svensson MK, et al. Clin Kidney J. 2024 Apr 4;17(8):sfae100. doi: 10.1093/ckj/sfae100. eCollection 2024 Aug. Clin Kidney J. 2024. PMID: 39165293 Free PMC article. - Dapagliflozin Improves Angiogenesis after Hindlimb Ischemia through the PI3K-Akt-eNOS Pathway.
Han L, Ye G, Su W, Zhu Y, Wu W, Hao L, Gao J, Li Z, Liu F, Duan J. Han L, et al. Biomolecules. 2024 May 16;14(5):592. doi: 10.3390/biom14050592. Biomolecules. 2024. PMID: 38785999 Free PMC article. - The Intertwined Relationship Between Heart Failure and Atrial Fibrillation, How Can We Untangle It?
Heo R. Heo R. Korean Circ J. 2024 May;54(5):268-269. doi: 10.4070/kcj.2024.0099. Korean Circ J. 2024. PMID: 38767339 Free PMC article. No abstract available. - Health and economic impact of dapagliflozin for type 2 diabetes patients who had or were at risk for atherosclerotic cardiovascular disease in the Italian general practitioners setting: a budget impact analysis.
Cortesi PA, Antonazzo IC, Palladino P, Gnesi M, Mele S, D'Amelio M, Zanzottera Ferrari E, Mazzaglia G, Mantovani LG. Cortesi PA, et al. Acta Diabetol. 2024 Aug;61(8):1017-1028. doi: 10.1007/s00592-024-02276-3. Epub 2024 Apr 18. Acta Diabetol. 2024. PMID: 38634912 Free PMC article.
References
- Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose‐lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006‐2013. Diabetologia. 2016;59(8):1692‐1701. - PubMed
- Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720‐1732. - PubMed
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. - PubMed
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous