Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway - PubMed (original) (raw)
doi: 10.1038/s41467-018-07859-7.
Sandeep Chandrashekharappa 2, Sobha R Bodduluri 1, Becca V Baby 1, Bindu Hegde 1, Niranjan G Kotla 2, Ankita A Hiwale 2, Taslimarif Saiyed 3, Paresh Patel 3, Matam Vijay-Kumar 4, Morgan G I Langille 5, Gavin M Douglas 5, Xi Cheng 4, Eric C Rouchka 6, Sabine J Waigel 7, Gerald W Dryden 7, Houda Alatassi 8, Huang-Ge Zhang 1, Bodduluri Haribabu 1, Praveen K Vemula 9, Venkatakrishna R Jala 10
Affiliations
- PMID: 30626868
- PMCID: PMC6327034
- DOI: 10.1038/s41467-018-07859-7
Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway
Rajbir Singh et al. Nat Commun. 2019.
Abstract
The importance of gut microbiota in human health and pathophysiology is undisputable. Despite the abundance of metagenomics data, the functional dynamics of gut microbiota in human health and disease remain elusive. Urolithin A (UroA), a major microbial metabolite derived from polyphenolics of berries and pomegranate fruits displays anti-inflammatory, anti-oxidative, and anti-ageing activities. Here, we show that UroA and its potent synthetic analogue (UAS03) significantly enhance gut barrier function and inhibit unwarranted inflammation. We demonstrate that UroA and UAS03 exert their barrier functions through activation of aryl hydrocarbon receptor (AhR)- nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent pathways to upregulate epithelial tight junction proteins. Importantly, treatment with these compounds attenuated colitis in pre-clinical models by remedying barrier dysfunction in addition to anti-inflammatory activities. Cumulatively, the results highlight how microbial metabolites provide two-pronged beneficial activities at gut epithelium by enhancing barrier functions and reducing inflammation to protect from colonic diseases.
Conflict of interest statement
V.R.J., P.K.V., H.B., R.S., S.C., and A.A.H. hold a patent application related to this technology (US patent application number: 62/671,737). P.K.V.’s interests were reviewed and are managed by the Institute for Stem Cell Biology and Regenerative Medicine (inStem) in accordance with their Conflict of Interest policies. The remaining authors declare no competing interests.
Figures
Fig. 1
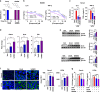
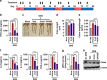
UAS03 is a potent anti-inflammatory structural analogue of UroA and induces tight junction proteins. a Chemical structures of UroA, UAS03. UroA/UAS03 stability was examined in the presence of gastric pH 2.0 and digestive enzymes. UroA and UAS03 (0.2 mg/ml) were incubated with digestive enzymes (esterases and proteases, 100 U/ml) for 12 h at 37 °C and compound levels were quantified. b BMDMs were stimulated with LPS (50 ng/ml) without or with UroA (blue line)/UAS03 (purple line) (0.1, 1, 10, 25, and 50 µM) for 6 h. IL-6 and TNF-α levels in supernatants were measured. c C57BL/6 mice (n = 3–4) were pretreated with UroA (20 mg/kg) and UAS03 (20 mg/kg). After 4 h, LPS (2 mg/kg) was injected intraperitoneally. Post 4 h of LPS administration, serum levels of IL-6 and TNF-α was measured. d–f HT29 or Caco2 cells were treated with vehicle (DMSO-0.01%) or UroA/UAS03 (50 μM) for 24 h. d The fold changes in mRNA levels of claudin 4 (Cldn4), occludin (Ocln), and Zona occludens 1 (ZO1) in HT29 cells were determined by RT PCR method. e UroA/UAS03 induced protein expression of Cldn4, Ocln, and ZO1 in HT29 cells were determined by immunoblots and quantified by Image J software. f Caco2 or HT29 cells were grown on coverslip bottom FluroDish and treated with Vehicle, UroA/UAS03 for 24 h. The cells were stained with anti-Cldn4 followed by secondary antibody tagged with Alexa-488. Nucleus was stained using DAPI. The confocal images were captured. The green intensity (n = 15–20 cell membrane regions) was measured. Scale bars for Caco2 and HT29 cells indicate 50 and 25 μm respectively. g Monolayer HT29 or Caco2 cells on transmembranes were treated with vehicle or UroA/UAS03 (50 μM) for 24 h followed by treatment with LPS (50 ng/ml) for 2 h. FITC-dextran was added to these cells (top of the membrane) and incubated for 2 h and FITC-dextran levels in bottom chamber well was measured. Results are representative of three independent experiments with triplicates for each concentration. *p < 0.05, **p < 0.01, ***p < 0.001, unpaired _t_-test between Veh, UroA, or UAS03. Error bars, ±SEM. Source Data are provided as a Source Data File
Fig. 2
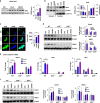
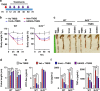
UroA/UAS03 enhance tight junction proteins in AhR-dependent manner. a HT29 cells were treated with vehicle (DMSO-0.01%)/UroA/UAS03 (50 μM) for 24 h. mRNA levels of Cytochrome P450 1A1 (Cyp1A1) was measured by RT PCR. b Cyp1A1 protein levels were measured using immunoblots and quantified band intensities by Image J software. c Cyp1A1 enzyme activity was measured by P450-Glo Cyp1A1 assay. HT29 cells were treated with UroA or UAS03 (0.1, 1, 10, 25, 50 µM) or FICZ (0.1, 1, 10, 25, 50 nM) for 24 h and enzyme Cyp1A1 activity was measured. d C57BL/6 and AhR−/− (n = 3) mice were treated orally with Vehicle (0.25% CMC), UroA or UAS03 (20 mg/kg) for 1 week and Cyp1A1 activity was measured in colons and livers by ethoxyresorufin-O-deethylase (EROD) assay. e The cells expressing AhR-reporter (luciferase) were treated with Veh or UroA/UAS03 or ellagic acid (EA) or MeBio (AhR high affinity ligand) for 6 h and fold change of luminescence over vehicle treatment was measured. f Immunofluorescence confocal images of HT29 cells treated with vehicle/UroA/UAS03 (50 μM) for 6 h. The cells were stained with anti-AhR antibody (red) and DAPI (blue). Relative fluorescence (n = ~20 cells) in the cytosol and nucleus was measured. The scale bar indicates 10 μm. g AhR levels in cytosol and nuclear fractions of HT29 cells treated for 2 h with Veh or UroA/UAS03 (50 μM). h AhR or i Cyp1A1 was knocked down using siRNA in HT29 cells and the cells were treated with vehicle/UroA/UAS03 (50 μM) for 24 h and immnunoblots were performed to detect expression of AhR, Cyp1A1, and Cldn4. Scrambled (Sc) siRNA transfections were used as controls. Immunoblots were quantified using Image J software. The data is representative of two independent repeats with triplicate wells for each treatment. Statistics performed using unpaired _t_-test using Graphpad Prism software. All in vitro studies were performed in triplicates. Error bars, ±SEM; ***p < 0.001; **p < 0.01; **p < 0.05. Source Data are provided as a Source Data File
Fig. 3
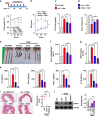
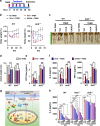
Nrf2 is required for UroA/UAS03 mediated upregulation of tight junction proteins. a Nrf2 levels were determined by immunoblots in HT29 cells treated with vehicle/UroA/UAS03 (50 μM) for 24 h. b Nrf2 expression in cytosolic and nuclear fractions of HT29 cells treated with Veh/UroA/UAS03 (50 μM) for 6 h. c Immunofluorescence confocal images of HT29 cells treated with vehicle/UroA/UAS03 (50 μM) for 6 h. The cells were stained with anti-Nrf2 antibody and DAPI. Relative green fluorescence (n = ~20 cells) intensity was measured. Scale bars indicate 25 μm. d Expression of Cldn4 and NQO1 in colon explants from WT, Nrf2−/−, and AhR−/− mice treated with vehicle/UroA/UAS03 (50 μM) for 24 h. Immunoblots were quantified using Image J software. e mRNA levels of Cldn4, Nrf2, and HO1 from colon explant cultures was measured by real-time PCR using SyBr green method. f C57BL/6, Nrf2−/−, and AhR−/− mice (n = 3) treated orally daily with veh or UroA/UAS03 (20 mg/kg) for 1 week. Cldn4 and NQO1 protein levels in colons were measured by immunoblots and quantified by Image J software. All in vitro studies were performed in triplicates. The immunoblots of colon explants and colon tissues were quantified from at least 6 independent runs. The levels of proteins were normalized to β-actin and Wild type vehicle treatment was set to 1 and calculated the fold changes. Statistics performed using unpaired _t_-test using Graphpad Prism software. Error bars, ±SEM; *p < 0.05; **p < 0.01; ***p < 0.001. Source Data are provided as a Source Data File
Fig. 4
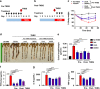
UroA/UAS03 treatment attenuates TNBS-induced colitis in mice. Colitis was induced by intrarectal administration of TNBS (2.5 mg/mouse) in C57BL/6 (8 week age old, n = 5/group) mice. Mice were orally treated with vehicle or UroA (20 mg/kg) or UAS03 (20 mg/kg body weight) every 12 h post-TNBS instillation for 60 h and the experiment terminated at 72 h. Representative data from one of three independent experiments is shown. a Percent body weight loss (No TNBS- Solid black line; Veh + TNBS- Solid red line; UroA + TNBS- Solid blue line; UAS03 + TNBS- Solid purple line). b disease activity index, c intestinal permeability, d colon lengths were measured. e Gross morphological changes of colon, f ratio of colon weight/length, g colonic myeloperoxidase (MPO) levels, h serum IL-6, TNF-α, CXCL1, and IL-1β levels, i microphotographs of hematoxylin and eosin (H&E) stained sections of colons and inflammation scores are shown. Scale bar indicates 300 μm. j Cldn4 expression in the colons of these mice (n = 3) was measured by immunoblots and quantified. Statistical analysis was performed (unpaired _t_-test) using Graphpad Prism software. Error bars, ±SEM ***p < 0.001; **p < 0.01 *p < 0.05. Source Data are provided as a Source Data File
Fig. 5
UroA/UAS03 prevent TNBS-induced colitis and sustain beneficial barrier activities. a Pre-TNBS treatment. Male C57BL/6 mice (n = 5 per group at 7–8 week old age) were given orally vehicle (Veh; 0.25% sodium carboxymethylcellulose) or UroA or UAS03 (20 mg/kg/bodyweight) daily for one week followed by rectal administration of TNBS to induce colitis. These mice did not receive any treatment post-TNBS administration. Mice were euthanized 72 h post-TNBS administration and characterized. b Post-TNBS treatment. Another set group of C57BL/6 mice (n = 5 per group at 7–8 week old age) received Veh or UroA or UAS03 (20 mg/kg) 24, 48, and 72 h post-TNBS. c Percent body weight loss was recorded after TNBS-administration. (No TNBS- Solid black line; Veh + TNBS- Solid red line; Pre-TNBS + UroA- Solid blue line; Pre-TNBS + UAS03- solid purple line; Post-TNBS + UroA- dashed blue line; Post-TNBS + UAS03- dashed purple line). d Representative colon images of control (no TNBS) along with vehicle/UroA/UAS03 treated mice from pre- and post-treatment groups. e Ratio of colon weight/length, f intestinal permeability was evaluated using FITC-dextran leakage assay. g Serum levels of IL-6 and TNF-α were measured using standard ELISA methods. Statistical analysis was performed (unpaired _t_-test) using Graphpad Prism software. Error bars, ±SEM ***p < 0.001. Source Data are provided as a Source Data File
Fig. 6
Treatment with UroA/UAS03 mitigate DSS-induced chronic colitis. a C57BL/6 mice (7–8 week age old) were treated with four cycles of DSS (2%) with 7 days/cycle with an interval of 14 days with regular water. Control group of mice (n = 5) received the regular water without DSS. UroA/UAS03 (20 mg/kg/day/body weight) that was resuspended in 0.25% sodium carboxymethylcellulose (CMC) solution (n = 9) or vehicle (CMC) (n = 9) was administered on 4th and 6th day of each DSS cycle and one treatment while on regular water. n = 5/control; n = 9/veh and UroA; n = 8/UAS03 group) Mice were euthanized at day 89 and the colitis phenotype was characterized. b Intestinal permeability using FITC-dextran was evaluated. c Representative colon images d colon lengths, e ratios of colon weight/length are shown. f Serum levels of IL-6, IL-1β, and TNF-α were measured using ELISA methods. g MPO levels were determined in colon tissues. h Cldn4 expression in the colons of these mice (n = 3) was measured by immunoblots. Statistics performed using unpaired _t_-test using Graphpad Prism software. Error bars, ±SEM ***p < 0.001; **p < 0.01; *p < 0.05. Source Data are provided as a Source Data File
Fig. 7
UroA/UAS03 utilize Nrf2 pathways to mitigate colitis. a–e Colitis was induced using TNBS in C57BL/6 (WT) and Nrf2−/− mice (n = 4–5/group 7–8 week old age). Mice were treated with Veh or UroA/UAS03 (20 mg/kg bodyweight) every 12 h post TNBS administration ending at 72 h. Representative data from two independent experiments is shown. a TNBS-induced colitis experimental design and treatment regimen. b Percent body weight loss (No TNBS- Solid black line; Veh + TNBS- Solid red line; UroA + TNBS- Solid blue line; UAS03 + TNBS- Solid purple line), c representative colon images, d colon lengths, e gut permeability, f serum levels of IL-6 and TNF-α were determined. Statistical analysis was performed (unpaired _t_-test) using Graphpad Prism software. Error bars, ±SEM ***p < 0.001; **p < 0.01 *p < 0.05. Source Data are provided as a Source Data File
Fig. 8
UroA/UAS03 exert beneficial activities through AhR-dependent pathways. a–e Colitis was induced using TNBS in C57BL/6 (WT) and AhR−/− mice (n = 4/group 7–8 week old age). Mice were treated with Veh or UroA/UAS03 (20 mg/kg bodyweight) every 12 h post TNBS administration and mice were euthanized at post 60 h TNBS administration. a TNBS-induced colitis experimental design and treatment regimen. b Percent body weight loss (No TNBS- Solid black line; Veh + TNBS- Solid red line; UroA + TNBS- Solid blue line; UAS03 + TNBS- Solid purple line), c representative colon images, d colon lengths, e gut permeability, f serum levels of IL-6 and TNF-α were determined. Statistical analysis was performed (unpaired _t_-test) using Graphpad Prism software. Error bars, ± SEM ***p < 0.001; **p < 0.01; *p < 0.05. g AhR-Nrf2 dependent tight junction protein regulation by UroA/UAS03. UroA/UAS03 (L:ligands) bind to AhR and activate its nuclear translocation to induce expression of Cyp1A1 and Nrf2. Further, UroA/UAS03 causes Nrf2-dependent upregulation of tight junction proteins and enhanced barrier function. h LPS (50 ng/ml)-induced IL-6 levels were measured in the presence of Vehicle or UroA or UAS03 (0.1, 1, 10, 20, 30, and 50 μM) in bone marrow derived macrophages (BMDM) from wild type (WT), Nrf2−/− and AhR−/− mice. The data is representative of two independent experiments with triplicates. Statistical analysis was performed (unpaired _t_-test) using Graphpad Prism software. Error bars, ±SEM ***p < 0.001; **p < 0.01 *p < 0.05. Source Data are provided as a Source Data File
Similar articles
- Cytochrome P450 1A1 is essential for the microbial metabolite, Urolithin A-mediated protection against colitis.
Ghosh S, Moorthy B, Haribabu B, Jala VR. Ghosh S, et al. Front Immunol. 2022 Sep 8;13:1004603. doi: 10.3389/fimmu.2022.1004603. eCollection 2022. Front Immunol. 2022. PMID: 36159798 Free PMC article. - Urolithin A-mediated augmentation of intestinal barrier function through elevated secretory mucin synthesis.
Yasuda T, Takagi T, Asaeda K, Hashimoto H, Kajiwara M, Azuma Y, Kitae H, Hirai Y, Mizushima K, Doi T, Inoue K, Dohi O, Yoshida N, Uchiyama K, Ishikawa T, Konishi H, Ukawa Y, Kohara A, Kudoh M, Inoue R, Naito Y, Itoh Y. Yasuda T, et al. Sci Rep. 2024 Jul 8;14(1):15706. doi: 10.1038/s41598-024-65791-x. Sci Rep. 2024. PMID: 38977770 Free PMC article. - AhR activation defends gut barrier integrity against damage occurring in obesity.
Postal BG, Ghezzal S, Aguanno D, André S, Garbin K, Genser L, Brot-Laroche E, Poitou C, Soula H, Leturque A, Clément K, Carrière V. Postal BG, et al. Mol Metab. 2020 Sep;39:101007. doi: 10.1016/j.molmet.2020.101007. Epub 2020 Apr 28. Mol Metab. 2020. PMID: 32360426 Free PMC article. - Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor.
Furue M, Tsuji G, Mitoma C, Nakahara T, Chiba T, Morino-Koga S, Uchi H. Furue M, et al. J Dermatol Sci. 2015 Nov;80(2):83-8. doi: 10.1016/j.jdermsci.2015.07.011. Epub 2015 Jul 26. J Dermatol Sci. 2015. PMID: 26276439 Review.
Cited by
- Correlation between intestinal microbiota and urolithin metabolism in a human walnut dietary intervention.
Liu H, Birk JW, Provatas AA, Vaziri H, Fan N, Rosenberg DW, Gharaibeh RZ, Jobin C. Liu H, et al. BMC Microbiol. 2024 Nov 15;24(1):476. doi: 10.1186/s12866-024-03626-5. BMC Microbiol. 2024. PMID: 39548408 - Inflammation-targeted delivery of Urolithin A mitigates chemical- and immune checkpoint inhibitor-induced colitis.
Ghosh S, Singh R, Goap TJ, Sunnapu O, Vanwinkle ZM, Li H, Nukavarapu SP, Dryden GW, Haribabu B, Vemula PK, Jala VR. Ghosh S, et al. J Nanobiotechnology. 2024 Nov 13;22(1):701. doi: 10.1186/s12951-024-02990-8. J Nanobiotechnology. 2024. PMID: 39533380 Free PMC article. - Parkinson's Disease and the Microbiota-Gut-Brain Axis: Metabolites, Mechanisms, and Innovative Therapeutic Strategies Targeting the Gut Microbiota.
Ran Z, Mu BR, Wang DM, Xin-Huang, Ma QH, Lu MH. Ran Z, et al. Mol Neurobiol. 2024 Nov 12. doi: 10.1007/s12035-024-04584-9. Online ahead of print. Mol Neurobiol. 2024. PMID: 39531191 Review. - Probiotic-derived extracellular vesicles alleviate AFB1-induced intestinal injury by modulating the gut microbiota and AHR activation.
Li J, Shi M, Wang Y, Liu J, Liu S, Kang W, Liu X, Chen X, Huang K, Liu Y. Li J, et al. J Nanobiotechnology. 2024 Nov 11;22(1):697. doi: 10.1186/s12951-024-02979-3. J Nanobiotechnology. 2024. PMID: 39529091 Free PMC article. - Gut microbiota and irritable bowel syndrome: status and prospect.
Cheng X, Ren C, Mei X, Jiang Y, Zhou Y. Cheng X, et al. Front Med (Lausanne). 2024 Oct 17;11:1429133. doi: 10.3389/fmed.2024.1429133. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39484201 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA216090/CA/NCI NIH HHS/United States
- R21CA216090 /NH/NIH HHS/United States
- P20 GM125504/GM/NIGMS NIH HHS/United States
- P20 GM103436/GM/NIGMS NIH HHS/United States
- R21 CA191683/CA/NCI NIH HHS/United States
- P30 GM106396/GM/NIGMS NIH HHS/United States
- P20GM125504-01 /NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases