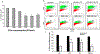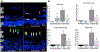Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion - PubMed (original) (raw)
Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion
Biji Mathew et al. Biomaterials. 2019 Mar.
Abstract
Retinal ischemia is a major cause of vision loss and impairment and a common underlying mechanism associated with diseases such as glaucoma, diabetic retinopathy, and central retinal artery occlusion. The regenerative capacity of the diseased human retina is limited. Our previous studies have shown the neuroprotective effects of intravitreal injection of mesenchymal stem cells (MSC) and MSC-conditioned medium in retinal ischemia in rats. Based upon the hypothesis that the neuroprotective effects of MSCs and conditioned medium are largely mediated by extracellular vesicles (EVs), MSC derived EVs were tested in an in-vitro oxygen-glucose deprivation (OGD) model of retinal ischemia. Treatment of R28 retinal cells with MSC-derived EVs significantly reduced cell death and attenuated loss of cell proliferation. Mechanistic studies on the mode of EV endocytosis by retinal cells were performed in vitro. EV endocytosis was dose- and temperature-dependent, saturable, and occurred via cell surface heparin sulfate proteoglycans mediated by the caveolar endocytic pathway. The administration of MSC-EVs into the vitreous humor 24 h after retinal ischemia in a rat model significantly enhanced functional recovery, and decreased neuro-inflammation and apoptosis. EVs were taken up by retinal neurons, retinal ganglion cells, and microglia. They were present in the vitreous humor for four weeks after intravitreal administration, with saturable binding to vitreous humor components. Overall, this study highlights the potential of MSC-EV as biomaterials for neuroprotective and regenerative therapy in retinal disorders.
Keywords: Apoptosis; Electroretinography; Endocytosis; Exosomes; Extracellular vesicles; Heparin sulfate proteoglycans; Inflammation; Ischemia; Mesenchymal stem cells; Microglia; Neuroprotection; Retinal ganglion cells; Vitreous humor binding.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement: None
Figures
Fig 1.. Characterization of MSC derived EVs.
(A) Nanoparticle Tracking Analysis (NTA) histogram demonstrating MSC-EVs’ size distribution after isolation using centrifugation and EV Exo-quick Isolation Reagent (see Methods for details). In the insert, mean and mode for particle size are displayed along with concentration. MSC-EVs showed a modal size of 93 nm, peaks at 89 and 141 nm (see Results for explanation), and the presence of few large vesicles (shown as larger peaks at higher diameters) indicating that the majority of the MSC-EVs are likely exosomes. (B) Western blot illustrating the characteristic surface markers of exosomes, CD63, CD9, CD81, and HSP70α, present in MSC-EV preparations, but not in MSC-conditioned medium (CM) depleted of EVs. Molecular weight markers are on left of each blot. (C) Transmission electron microscopic (TEM) image of cup-shaped MSC-EVs isolated from MSCs with diameters of approximately 100 nm, consistent with exosomal size. (D) Immunogold labeling of MSC-EVs with CD63 antibody to exosome surface markers, again demonstrating that the MSC-EVs are mainly exosomes. Scale bars are on lower left of Figs 1C and 1D.
Fig 2.
Endocytosis of MSC-EVs by R28 cells: (A) Representative confocal micrograph demonstrating endocytosis of fluorescently labeled EVs (green) by R28 cells. The cells were counter-stained with primary antibody to tubulin (cytoskeleton, red), and with DAPI to stain the nuclei (blue). Clockwise from top left are: DAPI (blue), MSC-EVs (green), composite of DAPI, MSCEVs, and tubulin (red). The image on top right of 2A demonstrates green punctae of MSC-EVs (light blue arrows) and denser concentrations of MSC-EVs (red arrows), and there is co-localization of MSC-EVs and tubulin within the cytoplasm of the cells (white arrows in lower right, composite panel of 2A). Scale bars are on the top of each panel. (B) Graph indicates a dose-dependent and saturable endocytosis of fluorescently labeled MSC-EVs. X-axis is volume of MSC-EVs and Y-axis indicates mean normalized fluorescence units. (C) Quantitative fluorescence measurements of MSC-EV endocytosis at 37°C and 4°C showing a decrease in endocytosis at lower temperature. Temperature is on X-axis, and Y-axis is mean normalized fluorescence units. The data represented in 2B and 2C are the mean of 6 individual experiments, and error bars indicate SD. * in Fig 2C represents statistical significance with respect to control (normothermia, P < 0.01).
Fig 3.. Heparan sulfate proteoglycans (HSPGs), but not integrins, are involved in endocytosis of MSC-EVs by R28 cells:
(A) Increasing doses of RGD peptide to block cell surface integrins did not alter endocytosis of fluorescently labeled MSC-EVs. Y-axis is mean normalized fluorescence units ± SD; the X-axis is dose of RGD in mM. No statistical significance was observed (n = 6 experiments). (B) Dose-dependent reduction of fluorescently labeled MSC-EV endocytosis after heparin pre-treatment to block HSPGs. Data on Y-axis is mean normalized fluorescence units ± SD; the X-axis is dose of heparin in μg/ml. * = P < 0.05 compared to vehicle (heparin = “0”), n = 6 experiments. (C) Representative confocal micrograph showing endocytosis of fluorescently labeled MSC-EVs by R28 cells treated with PBS vehicle (control). (D) Representative confocal micrograph showing no reduction in endocytosis of MSC-EVs after pre-incubation with RGD to block integrins (RGD = “0” is PBS vehicle alone). (E) Representative confocal micrograph showing reduction in endocytosis of MSC-EVs after they were pre-incubated with heparin to block HSPGs. (For C, D, and E, from left to right are shown MSCEVs (green), DAPI to stain the cell nuclei (blue), anti-tubulin to stain cytoskeleton (red), and composite of MSC-EVs, DAPI, and tubulin on the far right. Endocytosis can be seen in C and D, in the far right panels, where green MSC-EVs are visible inside cells (white arrows), as well as overlapping with tubulin (blue arrows). Scale bars appear on top or bottom of each panel.
Fig 4.. Involvement of the caveolar pathway in MSC-EV endocytosis by R28 cells:
(A) Representative confocal images showing endocytosed fluorescently labeled MSC-EVs (green) co-localized with anti-caveolin 1 (red). From left to right are DAPI (blue), MSC-EVs (green), cave-olin-1 (red), and merged. (B) Magnified area of box in A. White arrowheads point to regions of co-localization of caveolin-1 and MSC-EVs, as indicated by overlap of red and green to appear as yellow. (C) Representative confocal images of endocytosed MSC-EVs counterstained with anti-clathrin (red). From left to right are DAPI (blue), MSC-EVs (green), clathrin (red), and merged. (D) Magnified area of box in C. Note that in contrast to 4A-B, there is no co-localization of MSC-EVs and clathrin in C and in D. (E) Representative confocal images showing endocytosed fluorescently labeled MSC-EVs (green) in R28 cells. From left to right are DAPI (blue), MSC-EVs (green), anti-tubulin (red), and merged. MSC-EVs are visible inside the cells in the far right merged panel (green, shown by orange arrows), or yellow/orange, where tubulin (red) and MSC-EVs (green), co-localize (shown by white arrows). (F) Representative confocal images showing endocytosed fluorescently labeled MSC-EVs (green) in R28 cells after pre-treatment with methyl-β-cyclodextrin (MBCD) to disrupt R28 cell membrane cholesterol. From left to right are DAPI (blue), MSC-EVs (green), tubulin (red), and merged. (G) Quantitation of MBCD effect on endocytosis of MSC-EVs into R28 cells. There was a significant dose dependent reduction in MSC-EV uptake with increasing doses of MBCD. Data on the Y-axis is mean normalized fluorescence units ± SD; the X-axis is dose of MBCD in mM. * = P < 0.05 compared to control, n = 6 experiments.
Fig 5.. EVs protect retinal cells from OGD-induced cell death:
(A) Dose dependent effect of MSC-EVs on oxygen glucose deprivation (OGD) induced cytotoxicity of R28 cells as measured by lactate dehydrogenase (LDH) assay. Note the decrease in cell death from OGD with increasing dosage of MSC-EVs with saturation at 105 EVs/ml. In (A) data is presented as percentage cytotoxicity on Y-axis (% cell death, LDH, mean ± SD), and X-axis is concentration of MSCEVs in particles/ml. n = 6 experiments * = P < 0.05 vs OGD alone. (B) Representative flow cytometry results for the presence of EdU-positive cells after OGD with and without EVs. The percentages within the graphs in bold indicate the % of proliferating cells. Conditioned medium (CM) without EVs (CM-Exo), and PBS (ctrl) were controls. Exo = EVs. (C) Graphical representation of results in (B). Y-axis is % EdU-positive cells (mean ± SD). N = 4 experiments, * = p < 0.05 normoxia vs OGD, # = p < 0.05 vs control (“ctrl”, OGD + PBS). Both CM and Exo prevented the loss of proliferation in cells subjected to OGD, while CM-Exo showed no effect. Although there was a small decrease in proliferation in normoxic cells treated with EVs, there was no significant difference from controls.
Fig 6.. MSC-EVs enhance functional recovery after retinal ischemia in vivo.
Stimulus intensity plots of a-(A) and b-waves (B) were measured at baseline and at 8 days post ischemia. MSCEVs, PBS, or MSC medium depleted of EVs (EV depleted medium) were injected 24 h after ischemia into the vitreous humor of both eyes (right eye was ischemic and left eye was non ischemic control), as described in the methods section. (C) Representative ERG traces from ischemic retinae injected with PBS, MSC-EVs and medium depleted of EVs respectively; for brevity, only one set of representative traces, from ischemic eyes, per group is shown. The scale bars for amplitude (Y-axis, μV) and latency (X-axis, ms) appear in the top right of each representative ERG panel. N = 11–13 rats, for MSC-EVs or PBS; N = 6 for MSC-EV depleted medium. * = P < 0.05 for ischemic + MSC-EVs vs ischemic + PBS, # = P < 0.05 for medium depleted of MSC-EVs + ischemic vs MSC-EVs + ischemic.
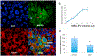
Fig 7.. MSC-EVs attenuated ischemia-induced apoptosis (TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay) in ischemic retinae in vivo.
(A) Representative immuno-histochemical images of TUNEL in retinal cryosections (7 μm) demonstrating MSC-EV-mediated reduction in TUNEL cells in ischemic retina compared to PBS injected ischemic. Red = TUNEL; Blue = DAPI, Green = fluorescently labeled MSC-EVs. In these experiments, the retinal cryosections were taken from retinae at 24 h after intravitreal injection of MSC-EVs or PBS, which was 48 h after ischemia. TUNEL cells are seen in the RGC layer (orange arrows), and in the inner (INL) and outer nuclear layers (ONL). (white arrows). IPL = inner plexiform layer. Note that aggregates of green MSC-EVs (yellow arrows) are present in the retinal ganglion cell (RGC) layer in EV ischemia (bottom right panel), and in the vitreous in EV control (bottom left panel). (B) Graphical representation of TUNEL cells in retinal ganglion cell layer, inner nuclear layer, outer nuclear layer, and total nuclei in retina, with data shown on Y-axis as TUNEL cells/20× field, mean ± SD. TUNEL was counted in all four groups (PBS control, MSC-EV control, PBS + ischemia and MSC-EVs + ischemia) by blinded observers. MSCEVs attenuated TUNEL in ischemic retinae, and there was no significant increase in TUNEL in normal eyes injected with MSC-EVs (“EV control”) except in the RGC layer. N = 4 rats per group; * = P < 0.05 for PBS non-ischemic vs PBS ischemic, or MSC-EV non ischemic vs MSCEV ischemic; # = P < 0.05 for PBS ischemic vs MSC-EV ischemic. ** = P < 0.05 for MSC-EV non-ischemic vs PBS non-ischemic.
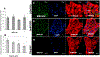
Fig 8.. MSC-EVs attenuated neuro-inflammation and caspase 3 activation after retinal ischemia in vivo:
(A) Representative Western blots for TNFα, IL-6 and cleaved caspase 3. β-Actin was used as the loading control. (B, C and D) are quantitative bar graphs of Western blots illustrating the significant MSC-EV-mediated amelioration of ischemia-induced increases in levels of inflammatory mediumtors (IL-6, TNFα), and apoptosis (cleaved caspase 3) in rats injected with intravitreal MSC-EVs 24 h after ischemia. There was no significant change in levels of IL-6, TNFα, or caspase 3 in MSC-EV injected normal eyes compared to PBS injected normal eyes. Retinal samples were collected 48 h after ischemia, which was 24 h after MSC-EV or PBS injection. N = 10 rats per group, * = P < 0.05 control non-ischemic vs ischemic, # = p < 0.05 PBS + ischemic vs MSC-EV + ischemic.
Fig 9.. In vivo live imaging of intra-vitreally injected green fluorescent MSC-EVs.
(A) Up-take of MSC-EVs into vitreous and retina of normal and ischemic eyes was imaged in real time by in vivo fundus imaging for a time course of four weeks (days 1 and 3, weeks 1, 2 and 4), using a Phoenix Micron IV (See Methods for more details). The control non-ischemic eyes are on the left and the ischemic on the right in each of the two columns in (A). Fluorescent MSC-EVs were present for up to 4 weeks after injection into the vitreous humor. Concentration of the MSC-EVs at the sites of injection into the vitreous and in the needle track likely explain the intense fluorescence in the day 1 and 3 images. (B) Graph representing binding of fluorescently labeled MSCEVs to 50 μg of isolated vitreous humor coated to 96-well assay plates. The binding of MSCEVs to the vitreous humor was saturable. Data points represent mean ± SD (n = 6 experiments) of normalized fluorescence intensity..
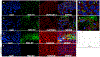
Fig 10.. Uptake and distribution of MSC-EVs by normal and ischemic retinae in vivo:
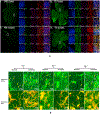
(A) Flat mount confocal microscopic imaging of retinae injected with fluorescent MSC-EVs (green) and stained with retinal markers anti-Brn-3a for retinal ganglion cells (RGCs, magenta), anti-Iba-1 for microglia (red) and nuclei (DAPI, blue). Representative images displayed for days 1, 3 and 7 for PBS-injected control (I) and ischemic (II) retinae (left panel) and MSC-EV injected control (III) and ischemic (IV) retinae (right panel). For each group a low magnification image is presented in the green channel indicating the overview of the flat mount. The square white box indicates the representative area shown under higher magnification. Higher magnification images (63×) are provided in all channels followed by a merged image for days 1 (A to E), 3 (F to J) and 7 (K to O). Comparing (III) and (IV), enhanced MSC-EV uptake can be seen in the ischemic (IV) compared to the normal retina (III), along with enhanced co-localization with the activated microglia (red). The composite images (E, J, and O) for each group show co-localization of MSC-EVs and microglia (yellow color, shown as white arrows in panel IVE), and Brn3a (white colored dots, shown by orange arrows in panel IVE), indicating that MSC-EVs were taken up by both RGCs and microglia after intravitreal administration. Blue arrows in panels IID and IVD show the greater amoeboid shape as opposed to ramified microglia indicating greater activation of microglia in ischemia-PBS injected compared to ischemia-MSC-EV injected retinae. N = 3 per time point. The uptake of MSC EVs by RGCs is further illustrated in (B), that are representative digital magnification of retinal flat mount images in (A) illustrating co-localization of MSC-EVs and distribution by specific retinal cell type in MSC-EV injected control and ischemic retinae, These images correspond to groups (III) and (IV) from (Fig 10A). Yellow arrows point to RGCs co-localized with MSC-EVs (green and magenta forming white, or white together with magenta, as Brn3a is a nuclear protein) and red arrows point to MSC-EVs with microglial cells (green and red forming yellow or orange).
Fig 11.. High magnification confocal imaging of retinal flat mounts shows that retinal neurons and retinal ganglion cells take up MSC-EVs, and that ischemia increases uptake.
Top panel shows control, non-ischemic retina, and bottom panel shows ischemic retina. Retinal flat mounts of non-ischemic eyes injected with green-labeled MSC-EVs, stained for (A) DAPI (blue), (B) EVs alone (green), (C) Beta-tubulin III alone (βT3, red), and (D) Brn-3a alone (magenta), (E) EVs (green) + βT3 (red), and F) EVs (green) + Brn-3a (magenta). βT3 stains only neurons and their axonal or dendritic projections. These flat mounts are from retinas harvested 24 h after injection of MSC-EVs, which was 48 h after ischemia. Red arrows in F) indicate the presence of EVs within the cell body of the retinal ganglion cells (Brn-3a stains only the nuclei of RGCs). Note that the majority of cells in (B), (E), and (F) show punctate green staining indicating that EVs were taken up by the cells. White arrows in (E) show the co-localization between the MSC-EVs and the retinal neuron cell bodies, indicated by the orange-yellow or orange color. White arrowheads mark the axonal or dendritic projections of the retinal neurons, and the presence therein of MSC-EVs (E).
Similar articles
- MicroRNA-based engineering of mesenchymal stem cell extracellular vesicles for treatment of retinal ischemic disorders: Engineered extracellular vesiclesand retinal ischemia.
Mathew B, Acha LG, Torres LA, Huang CC, Liu A, Kalinin S, Leung K, Dai Y, Feinstein DL, Ravindran S, Roth S. Mathew B, et al. Acta Biomater. 2023 Mar 1;158:782-797. doi: 10.1016/j.actbio.2023.01.014. Epub 2023 Jan 11. Acta Biomater. 2023. PMID: 36638942 Free PMC article. - Uptake and Distribution of Administered Bone Marrow Mesenchymal Stem Cell Extracellular Vesicles in Retina.
Mathew B, Torres LA, Gamboa Acha L, Tran S, Liu A, Patel R, Chennakesavalu M, Aneesh A, Huang CC, Feinstein DL, Mehraeen S, Ravindran S, Roth S. Mathew B, et al. Cells. 2021 Mar 25;10(4):730. doi: 10.3390/cells10040730. Cells. 2021. PMID: 33806128 Free PMC article. - Extracellular Vesicles Derived from Wharton's Jelly Mesenchymal Stem Cells Prevent and Resolve Programmed Cell Death Mediated by Perinatal Hypoxia-Ischemia in Neuronal Cells.
Joerger-Messerli MS, Oppliger B, Spinelli M, Thomi G, di Salvo I, Schneider P, Schoeberlein A. Joerger-Messerli MS, et al. Cell Transplant. 2018 Jan;27(1):168-180. doi: 10.1177/0963689717738256. Cell Transplant. 2018. PMID: 29562785 Free PMC article. - Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications.
Tsiapalis D, O'Driscoll L. Tsiapalis D, et al. Cells. 2020 Apr 16;9(4):991. doi: 10.3390/cells9040991. Cells. 2020. PMID: 32316248 Free PMC article. Review. - Functional proteins of mesenchymal stem cell-derived extracellular vesicles.
Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Qiu G, et al. Stem Cell Res Ther. 2019 Nov 28;10(1):359. doi: 10.1186/s13287-019-1484-6. Stem Cell Res Ther. 2019. PMID: 31779700 Free PMC article. Review.
Cited by
- Emerging role of extracellular vesicles in diabetic retinopathy.
Zhu J, Huang J, Sun Y, Xu W, Qian H. Zhu J, et al. Theranostics. 2024 Feb 4;14(4):1631-1646. doi: 10.7150/thno.92463. eCollection 2024. Theranostics. 2024. PMID: 38389842 Free PMC article. Review. - Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms.
Zhao Y, Zhu X, Zhang L, Ferguson CM, Song T, Jiang K, Conley SM, Krier JD, Tang H, Saadiq I, Jordan KL, Lerman A, Lerman LO. Zhao Y, et al. Stem Cells Dev. 2020 Sep 15;29(18):1190-1200. doi: 10.1089/scd.2020.0030. Epub 2020 Aug 3. Stem Cells Dev. 2020. PMID: 32657229 Free PMC article. - Bone regeneration is mediated by macrophage extracellular vesicles.
Kang M, Huang CC, Lu Y, Shirazi S, Gajendrareddy P, Ravindran S, Cooper LF. Kang M, et al. Bone. 2020 Dec;141:115627. doi: 10.1016/j.bone.2020.115627. Epub 2020 Sep 3. Bone. 2020. PMID: 32891867 Free PMC article. - Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5.
Liu Y, Guo Y, Bao S, Huang H, Liu W, Guo W. Liu Y, et al. Cell Death Dis. 2022 Mar 28;13(3):278. doi: 10.1038/s41419-022-04703-1. Cell Death Dis. 2022. PMID: 35351860 Free PMC article. - Neuroprotective mechanism of crocin via PI3K/Akt/mTOR signaling pathway after cerebral infarction: an in vitro study.
Zhao T, Lu H, Li M, Yan Q, Gu J, Liu L. Zhao T, et al. Am J Transl Res. 2022 May 15;14(5):3164-3171. eCollection 2022. Am J Transl Res. 2022. PMID: 35702067 Free PMC article.
References
- Roth S, Dreixler JC, Mathew B, Balyasnikova I, Mann JR, Boddapati V, Xue L, Lesniak MS, Hypoxic-Preconditioned Bone Marrow Stem Cell Medium Significantly Improves Outcome After Retinal Ischemia in RatsPreconditioned Stem Cells and Retinal Ischemia, Investigative ophthalmology & visual science 57(7) (2016) 3522–3532. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY001792/EY/NEI NIH HHS/United States
- R01 DE027404/DE/NIDCR NIH HHS/United States
- R56 DE023806/DE/NIDCR NIH HHS/United States
- R01 EY010343/EY/NEI NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- KL2 TR000048/TR/NCATS NIH HHS/United States
- R21 EY028690/EY/NEI NIH HHS/United States
- R01 EY026286/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources