Oleuropein derivatives from olive fruit extracts reduce α-synuclein fibrillation and oligomer toxicity - PubMed (original) (raw)
. 2019 Mar 15;294(11):4215-4232.
doi: 10.1074/jbc.RA118.005723. Epub 2019 Jan 17.
Farhang Aliakbari 2 3, Cagla Sahin 2 4, Charlotte Lomax 5, Ahmed Tawfike 5, Nicholas P Schafer 2, Alireza Amiri-Nowdijeh 6, Hoda Eskandari 2, Ian Max Møller 4, Mehdi Hosseini-Mazinani 6, Gunna Christiansen 7, Jane L Ward 5, Dina Morshedi 8, Daniel E Otzen 9 10
Affiliations
- PMID: 30655291
- PMCID: PMC6422090
- DOI: 10.1074/jbc.RA118.005723
Oleuropein derivatives from olive fruit extracts reduce α-synuclein fibrillation and oligomer toxicity
Hossein Mohammad-Beigi et al. J Biol Chem. 2019.
Abstract
Aggregation of α-synuclein (αSN) is implicated in neuronal degeneration in Parkinson's disease and has prompted searches for natural compounds inhibiting αSN aggregation and reducing its tendency to form toxic oligomers. Oil from the olive tree (Olea europaea L.) represents the main source of fat in the Mediterranean diet and contains variable levels of phenolic compounds, many structurally related to the compound oleuropein. Here, using αSN aggregation, fibrillation, size-exclusion chromatography-multiangle light scattering (SEC-MALS)-based assays, and toxicity assays, we systematically screened the fruit extracts of 15 different olive varieties to identify compounds that can inhibit αSN aggregation and oligomer toxicity and also have antioxidant activity. Polyphenol composition differed markedly among varieties. The variety with the most effective antioxidant and aggregation activities, Koroneiki, combined strong inhibition of αSN fibril nucleation and elongation with strong disaggregation activity on preformed fibrils and prevented the formation of toxic αSN oligomers. Fractionation of the Koroneiki extract identified oleuropein aglycone, hydroxyl oleuropein aglycone, and oleuropein as key compounds responsible for the differences in inhibition across the extracts. These phenolic compounds inhibited αSN amyloidogenesis by directing αSN monomers into small αSN oligomers with lower toxicity, thereby suppressing the subsequent fibril growth phase. Our results highlight the molecular consequences of differences in the level of effective phenolic compounds in different olive varieties, insights that have implications for long-term human health.
Keywords: Mediterranean diet; Parkinson disease; amyloid; cell toxicity; membrane permeabilization; neurodegeneration; oligomerization; olive polyphenols; protein aggregation; α-synuclein (α-synuclein).
© 2019 Mohammad-Beigi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Figure 1.
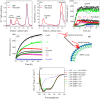
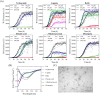
The effect of the extracts on αSN fibrillation. A, the effect of 0–0.3 mg/ml of the olive extracts on the kinetics of αSN fibrillation at 5 different concentrations monitored by ThT fluorescence. Joined lines show the Finke-Watzky model fitted to the experimental data. B, the effect of the selected extracts (7 of 15 extracts) at different concentrations (0.025, 0.05, 0.1, 0.15, and 0.3 mg/ml) on the maximum ThT fluorescence intensity. C, ThT end point levels are converted to % inhibition and fitted to Equation 1 to calculate the IC50 of the extracts. D–F, kinetic parameters for αSN fibrillation as a function of various concentrations of the extracts relative to the values in the absence of the extracts (D, relative growth rate (ν/ν,control); E, relative half-time (_t_½/_t_½, control), and F, relative lag time (tN/tN, control)).
Figure 2.
Far-UV CD spectra of αSN incubated alone (control) and in the presence of (A) 0.3 mg/ml, (B) 0.15 mg/ml, and (C) 0.1 mg/ml of the 7 selected extracts after 24 h. D, normalized β structure (%) of αSN incubated in the presence of the 7 selected extracts. E, SDS-PAGE analysis of the supernatants of the incubated samples of αSN in the absence and presence of 0.15 and 0.3 mg/ml of the best extracts in inhibiting αSN fibrillation. Monomeric αSN has a molecular mass of 14.5 kDa. Arrows highlight dimers (≈35 kDa) and oligomers (>250 kDa). F, the SEC-profile of the supernatants of the samples of αSN incubated for 24 h with or without 0.15 mg/ml of Koroneiki extract. G, EM of αSN after 24 h incubation in the absence (control) and presence of 0.3 mg/ml of the 7 selected olive fruit extracts. Scale bar, 200 nm. The size and distribution of the fibrils in each sample are summarized in the table below G. The length of fibrils were obtained in three TEM images for each sample and averaged.
Figure 3.
The SEC-profile of the supernatants of the (A) samples of αSN incubated for 1 h and (B) preformed αSN fibrils preincubated overnight with or without 0.15 mg/ml of Koroneiki extract. The effect of the extracts (0.15 mg/ml) on the seeding of αSN aggregation under shaking (C) and nonshaking (D) conditions. E, schematic representation of the α-helix structure induced in αSN by DMPG vesicles. F, the effect of the extracts (0.15 mg/ml) on the interaction of monomer (14 μ
m
) and DMPG vesicles (0.2 mg/ml).
Figure 4.
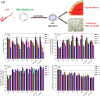
A, schematic representation of the effect of olive variety extracts on the membrane permeabilization and cytotoxicity of αSN aggregates. B, calcein release from DOPG vesicles after 20 min incubation with αSN aggregates formed alone and in the presence of the best extracts (0.3 mg/ml) over different times (0–24 h). Viability of OLN-93 (C) and SH-SY5Y (D) cells after 2–24 h incubation with αSN aggregates formed alone and in the presence of the best extracts (0.3 mg/ml) over different times (0–24 h). E, cytotoxicity of αSN aggregates to SH-SY5Y cells was assayed by LDH release. LDH signals were normalized to untreated cells. For all assays, values represent mean ± S.D. and the differences between the groups and αSN control are significant (p < 0.05) unless marked ns.
Figure 5.
A, schematic representation of the effect of Koroneiki extract fractions on the membrane permeabilization and cytotoxicity of αSN aggregates. B, calcein release from DOPG vesicles induced by oligomers (0.1 μ
m
) either alone or in the presence of Koroneiki (0.2 mg/ml). The Koroneiki extract is used at the highest concentration at which quenching of fluorescence does not happen. C, viability of SH-SY5Y cells incubated with αSN oligomers with or without co-incubation with Koroneiki (0.15 mg/ml).
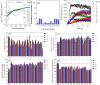
Figure 6.
A, far-UV CD spectra of αSN incubated alone (control) and in the presence of 3 mg/ml of Koroneiki extract fractions. B, normalized β structure (%) of αSN incubated in the presence of Koroneiki extract fractions. C, the effect of the Koroneiki extract fractions (1.5 mg/ml) on the seeding of αSN aggregation under shaking conditions. D, calcein release from DOPG vesicles after 20 min incubation with αSN aggregates formed alone and in the presence of Koroneiki extract fractions (3 mg/ml) over different times (0–24 h). Viability of OLN-93 (E) and SH-SY5Y (F) cells after 24 h incubation with αSN aggregates were formed alone and in the presence of Koroneiki extract fractions (3 mg/ml) over different times (0–24 h). G, cytotoxicity of αSN aggregates to SH-SY5Y cells was assayed by LDH release. LDH signals were normalized to untreated cells. For all assays, values represent mean ± S.D. and the differences between the groups and αSN control are significant (p < 0.05), unless indicated ns.
Figure 7.
The effect of Koroneiki compounds on fibrillation of 1 mg/ml αSN. A, the kinetics of αSN fibrillation in the presence of 0–200 μ
m
Koroneiki compounds, monitored by ThT fluorescence. B, far-UV CD spectra of αSN incubated alone (Ctrl) and in the presence of 200 μ
m
Koroneiki compounds after 24 h. C, EM of αSN after 24 h incubation in the presence of 200 μ
m
oleuropein. Scale bar, 200 nm.
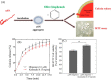
Figure 8.
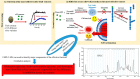
Schematic representation of screening of different olive varieties shown in panel 1. The first screening of fruit extract of 15 different olive varieties on the kinetic analysis of αSN fibrillation is shown in panel 2. The most effective variety, Koroneiki, combined strong inhibition of αSN fibril nucleation and elongation with a strong ability to disaggregate preformed fibrils and prevent formation of toxic αSN oligomers is shown in panel 3. Koroneiki fruit extract was fractionated and by using the same assay as the ones used in step 2, the most effective fractions were identified. LS-MS analysis was further used to identify the major compounds in the effective fractions. Correlation analysis confirmed oleuropein aglycone, hydroxyl oleuropein aglycone, and oleuropein as key compounds responsible for the difference in inhibition across the extracts.
Similar articles
- Potent α-Synuclein Aggregation Inhibitors, Identified by High-Throughput Screening, Mainly Target the Monomeric State.
Kurnik M, Sahin C, Andersen CB, Lorenzen N, Giehm L, Mohammad-Beigi H, Jessen CM, Pedersen JS, Christiansen G, Petersen SV, Staal R, Krishnamurthy G, Pitts K, Reinhart PH, Mulder FAA, Mente S, Hirst WD, Otzen DE. Kurnik M, et al. Cell Chem Biol. 2018 Nov 15;25(11):1389-1402.e9. doi: 10.1016/j.chembiol.2018.08.005. Epub 2018 Sep 6. Cell Chem Biol. 2018. PMID: 30197194 - Inhibition of human islet amyloid polypeptide aggregation and cellular toxicity by oleuropein and derivatives from olive oil.
Chaari A. Chaari A. Int J Biol Macromol. 2020 Nov 1;162:284-300. doi: 10.1016/j.ijbiomac.2020.06.170. Epub 2020 Jun 20. Int J Biol Macromol. 2020. PMID: 32569693 - How epigallocatechin gallate binds and assembles oligomeric forms of human alpha-synuclein.
Andersen CB, Yoshimura Y, Nielsen J, Otzen DE, Mulder FAA. Andersen CB, et al. J Biol Chem. 2021 Jan-Jun;296:100788. doi: 10.1016/j.jbc.2021.100788. Epub 2021 May 18. J Biol Chem. 2021. PMID: 34019875 Free PMC article. - Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L.
Romani A, Ieri F, Urciuoli S, Noce A, Marrone G, Nediani C, Bernini R. Romani A, et al. Nutrients. 2019 Aug 1;11(8):1776. doi: 10.3390/nu11081776. Nutrients. 2019. PMID: 31374907 Free PMC article. Review. - Polyphenols benefits of olive leaf (Olea europaea L) to human health.
Vogel P, Kasper Machado I, Garavaglia J, Zani VT, de Souza D, Morelo Dal Bosco S. Vogel P, et al. Nutr Hosp. 2014 Dec 17;31(3):1427-33. doi: 10.3305/nh.2015.31.3.8400. Nutr Hosp. 2014. PMID: 25726243 Review.
Cited by
- Mechanistic Insights into Polyphenols' Aggregation Inhibition of α-Synuclein and Related Peptides.
Martins GF, Nascimento C, Galamba N. Martins GF, et al. ACS Chem Neurosci. 2023 May 17;14(10):1905-1920. doi: 10.1021/acschemneuro.3c00162. Epub 2023 Apr 26. ACS Chem Neurosci. 2023. PMID: 37125909 Free PMC article. - Enhanced accumulation of reduced glutathione by Scopoletin improves survivability of dopaminergic neurons in Parkinson's model.
Pradhan P, Majhi O, Biswas A, Joshi VK, Sinha D. Pradhan P, et al. Cell Death Dis. 2020 Sep 10;11(9):739. doi: 10.1038/s41419-020-02942-8. Cell Death Dis. 2020. PMID: 32913179 Free PMC article. - The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile.
Fatima MT, Bhat AA, Nisar S, Fakhro KA, Al-Shabeeb Akil AS. Fatima MT, et al. Heliyon. 2022 Dec 30;9(1):e12698. doi: 10.1016/j.heliyon.2022.e12698. eCollection 2023 Jan. Heliyon. 2022. PMID: 36632095 Free PMC article. Review. - Glycation modulates alpha-synuclein fibrillization kinetics: A sweet spot for inhibition.
Farzadfard A, König A, Petersen SV, Nielsen J, Vasili E, Dominguez-Meijide A, Buell AK, Outeiro TF, Otzen DE. Farzadfard A, et al. J Biol Chem. 2022 May;298(5):101848. doi: 10.1016/j.jbc.2022.101848. Epub 2022 Mar 18. J Biol Chem. 2022. PMID: 35314196 Free PMC article. - Natural Phenolic Compounds with Neuroprotective Effects.
Tavan M, Hanachi P, de la Luz Cádiz-Gurrea M, Segura Carretero A, Mirjalili MH. Tavan M, et al. Neurochem Res. 2024 Feb;49(2):306-326. doi: 10.1007/s11064-023-04046-z. Epub 2023 Nov 8. Neurochem Res. 2024. PMID: 37940760 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources