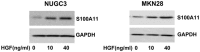HGF-mediated S100A11 overexpression enhances proliferation and invasion of gastric cancer - PubMed (original) (raw)
. 2018 Nov 15;10(11):3385-3394.
eCollection 2018.
Affiliations
- PMID: 30662594
- PMCID: PMC6291695
HGF-mediated S100A11 overexpression enhances proliferation and invasion of gastric cancer
Sung Ae Koh et al. Am J Transl Res. 2018.
Abstract
S100 proteins are a group of low molecular weight (10-12 kDa) acidic proteins belonging to the largest family of EFhand calciumbinding proteins. S100A11, also known as S100C or calgizzarin, is an important member of the S100 family. S100A11 overexpression has been reported in a number of cancers including papillary thyroid carcinoma, colon, pancreatic, and breast cancer. One other study demonstrated that increased S100A11 expression is correlated with gastric cancer metastasis and poor overall disease prognosis. This study aimed to identify the function of S100A11 associated with cell proliferation and invasion in gastric cancer. We used cell culture, western blotting, reverse transcription-polymerase chain reaction, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and S100A11 knock-down with short hairpin ribonucleic acid (shRNA). First, we confirmed that the S100A11 expression was upregulated by hepatocyte growth factor (HGF). The role of S100A11 was determined via knock down of S100A11. S100A11-shRNA cells showed decreased levels of metalloproteinase-9 (MMP9) and nuclear factor kappa-B (NF-κB). We also examined and confirmed the role of HGF-mediated S100A11 expression. HGF-mediated cell proliferation and in vitro invasion increased, and HGF-mediated apoptosis decreased in S100A11 knockdown cells. We identified the putative binding site of NF-κB in the MMP9 promoter region and confirmed its function via chromatin immunoprecipitation (CHIP) assay. Our results showed that S100A11 is upregulated by HGF through the NF-κB pathway in gastric cancer and plays a role in cell proliferation and invasion in gastric cancer. It may thus be a possible target for gastric cancer therapy.
Keywords: HGF; S100A11; gastric cancer; invasion.
Conflict of interest statement
None.
Figures
Figure 1
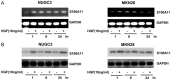
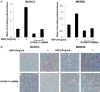
Overexpression of S100A11 level with HGF in NUGC-3 and MKN-28 cells. Cells were serum-starved for 24 h, treated with/without HGF (10 ng/mL) for the indicated times, and harvested. The expression levels of S100A11 RNA and protein were confirmed by RT-PCR (A) and western blot analysis (B). Representative data from three independent experiments are shown. HGF, hepatocyte growth factor; RNA, ribonucleic acid; RT-PCR, reverse transcription-polymerase chain reaction.
Figure 2
Dose effects of HGF on S100A11 expression in NUGC-3 and MKN-28 cells. Cells were serum-starved for 24 h, treated with HGF (0, 10 and 40 ng/ml) for 1 h, and harvested. S100A11 expression levels were confirmed by western blotting. Representative data from three independent experiments are shown. HGF, hepatocyte growth factor.
Figure 3
Overexpression of MMP9 level with HGF in NUGC-3 and MKN-28 cells. Cells were serum-starved for 24 h, treated with HGF (0, 10 and 40 ng/ml) for 1 h, and harvested. The MMP-9 protein levels in culture media were analysed by western blotting (A) and zymography (B). Representative data from three independent experiments are shown. MMP9, metalloproteinase-9.
Figure 4
Effects of LY, PD, SB on the S100A11 and NF-κB expression. The treatment of LY showed the decreased level in both S100A11 and NF-κB expression level in both NUGC-3 and MKN-28 cell lines. The cells (1 × 106/well) were plated overnight in complete medium, starved for 24 h, and then treated with or without LY, PD, SB does dependent for 1 h prior to incubation with or without 10 ng/mL of HGF and harvested. The LCN2 expression was analyzed by Western blotting. This illustrates representative data from three independent experiments. NF-κB, nuclear factor kappa-B; HGF, hepatocyte growth factor.
Figure 5
Down regulation of S100A11 and MMP-9 expression with LY. Cells (1 × 106/well) were plated overnight in complete medium, starved for 24 h, and then treated with or without LY at different doses for 1 h prior to incubation with or without 10 ng/ml of HGF for 48 h, and then harvested. S100A11 expression was analyzed by western blotting. MMP-9 secreted in the media was analyzed by western blotting and zymography. Representative data from three independent experiments are shown. MMP, metalloproteinase; HGF, hepatocyte growth factor.
Figure 6
Effects of NF-κB on S100A11 expression. The expression level of S100A11 was decreased with PDTC treatment. Serum-starved cells were pre-treated with different doses of LY294002 for 45 min, incubated with 10 ng/ml of HGF for 15 min, and harvested. The NF-κB expression levels were confirmed by western blotting (A, B). Serum-starved cells were pretreated with/without PDTC (100 µM) for 45 min, then incubated with 10 ng/mL of HGF for 15 min, and harvested. LCN2 expression levels were confirmed by western blotting (C). Representative data from three independent experiments are shown. NF-κB, nuclear factor kappa-B; HGF, hepatocyte growth factor; PDTC, pyrrolidine dithiocarbamate; LCN2, lipocalin 2.
Figure 7
Down regulation of MMP9, NF-κB protein expression in S100A11 knockdown cells. Control cells and stable S100A11-shRNA cells (1 × 106/well) were plated overnight in complete medium, starved for 24 h, treated with/without 10 ng/mL HGF for 1 h, and harvested. For MMP9 analysis, control cells and stable S100A11-shRNA cells (1 × 106/well) were plated overnight in complete medium, starved for 24 h, treated with/without 10 ng/ml HGF for 48 h, and the spent medium was harvested. The expression levels of MMP9, NF-κB, and S100A11 were analyzed by western blotting. Representative data from three independent experiments are shown. MMP9, metalloproteinase-9; NF-κB, nuclear factor kappa-B; shRNA, short hairpin ribonucleic acid.
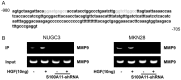
Figure 8
Effect S100A11 knockdown on MMP9 promoter activation by HGF. CHIP assay results show the amplification of a fragment of the proximal MMP9 promoter containing the NF-κB binding site but not observed the amplification in S100A11 knockdown cells. Immunoprecipitation was carried out using an anti-NF-κB antibody. Representative data from 3 independent experiments are shown. MMP9, metalloproteinase-9; HGF, hepatocyte growth factor; CHIP, chromatin immunoprecipitation; NF-κB, nuclear factor kappa-B.
Figure 9
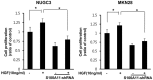
Down regulation of S100A11 knockdown on cell proliferation. Control cells (1,000/well) and stable S100A11-shRNA cells were seeded in 96-well plates with DMEM supplemented with 5% FBS and incubated for 24 h. After serum-starvation for 24 h, cells were treated with/without 10 ng/mL HGF for 72 h. Cell proliferation was measured using MTT assays and expressed as a percentage of HGF-untreated control cells. Values are means ± SD of three independent experiments performed in triplicate. shRNA, short hairpin ribonucleic acid; DMEM, Dulbecco’s Modified Eagle’s Medium; FBS, fetal bovine serum; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; HGF, hepatocyte growth factor; SD, standard deviation.
Figure 10
Down regulation of S100A11 knockdown on HGF-mediated cell invasion. Stable S100A11-shRNA cells and control cells were treated with/without 10 ng/mL HGF for 48 h. Cell invasion capacity was measured using the standard two chamber invasion assay with Matrigel-coated migration chambers. Values are means ± SD of three independent experiments. HGF, hepatocyte growth factor; shRNA, short hairpin ribonucleic acid; SD, standard deviation.
Similar articles
- The Important Role of GPX1 and NF-κB Signaling Pathway in Human Gastric Cancer: Implications for Cell Proliferation and Invasion.
Jang BI, Jung JY, Koh SA, Lee KH. Jang BI, et al. Cancer Genomics Proteomics. 2024 Apr 26;21(3):305-315. doi: 10.21873/cgp.20449. Cancer Genomics Proteomics. 2024. PMID: 38670589 Free PMC article. - BAG3 contributes to HGF-mediated cell proliferation, migration, and invasion via the Egr1 pathway in gastric cancer.
Lee JC, Koh SA, Lee KH, Kim JR. Lee JC, et al. Tumori. 2019 Feb;105(1):63-75. doi: 10.1177/0300891618811274. Epub 2018 Dec 4. Tumori. 2019. PMID: 30514177 - HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-κB activation.
Koh SA, Lee KH. Koh SA, et al. Oncol Rep. 2015 Oct;34(4):2179-87. doi: 10.3892/or.2015.4189. Epub 2015 Aug 10. Oncol Rep. 2015. PMID: 26259977 - The Calcium Binding Protein S100A11 and Its Roles in Diseases.
Zhang L, Zhu T, Miao H, Liang B. Zhang L, et al. Front Cell Dev Biol. 2021 Jun 11;9:693262. doi: 10.3389/fcell.2021.693262. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34179021 Free PMC article. Review.
Cited by
- Lidocaine suppresses the malignant behavior of gastric cancer cells via the c-Met/c-Src pathway.
Zeng W, Xing ZT, Tan MY, Wu YW, Zhang CY. Zeng W, et al. Exp Ther Med. 2021 May;21(5):424. doi: 10.3892/etm.2021.9868. Epub 2021 Feb 25. Exp Ther Med. 2021. PMID: 33747163 Free PMC article. - Inhibition of lung cancer by vitamin D depends on downregulation of histidine-rich calcium-binding protein.
Liu N, Li X, Fu Y, Li Y, Lu W, Pan Y, Yang J, Kong J. Liu N, et al. J Adv Res. 2020 Aug 27;29:13-22. doi: 10.1016/j.jare.2020.08.013. eCollection 2021 Mar. J Adv Res. 2020. PMID: 33842001 Free PMC article. - DNA Methylation-based Diagnostic and Prognostic Biomarkers for Glioblastoma.
Tang Y, Qing C, Wang J, Zeng Z. Tang Y, et al. Cell Transplant. 2020 Jan-Dec;29:963689720933241. doi: 10.1177/0963689720933241. Cell Transplant. 2020. PMID: 32510239 Free PMC article. - Interleukin 6 regulates the expression of programmed cell death ligand 1 in thyroid cancer.
Zhang GQ, Jiao Q, Shen CT, Song HJ, Zhang HZ, Qiu ZL, Luo QY. Zhang GQ, et al. Cancer Sci. 2021 Mar;112(3):997-1010. doi: 10.1111/cas.14752. Epub 2021 Feb 24. Cancer Sci. 2021. PMID: 33247999 Free PMC article. - Exploring the prognostic and diagnostic value of lactylation-related genes in sepsis.
Li S, Shen Y, Wang C, Yang J, Chen M, Hu Y. Li S, et al. Sci Rep. 2024 Oct 4;14(1):23130. doi: 10.1038/s41598-024-74040-0. Sci Rep. 2024. PMID: 39367086 Free PMC article.
References
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. - PubMed
- Cross S, Hamdy F, Deloulme JC, Rehman I. Expression of S100 proteins in normal human tissues and common cancers using tissue microarrays: S100A6, S100A8, S100A9 and S100A11 are all overexpressed in common cancers. Histopathology. 2005;46:256–269. - PubMed
- Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. - PubMed
- Feng G, Xu U, Youssef EM, Lotan R. Diminished expression of S100A2, a putative tumor suppressor, at early stage of human lung carcinogenesis. Cancer Res. 2001;61:7999–8004. - PubMed
- Wicki R, Franz C, Scholl FA, Heizmann CW, Schäfer BW. Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell Calcium. 1997;22:243–254. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous