Stem cells from human exfoliated deciduous teeth correct the immune imbalance of allergic rhinitis via Treg cells in vivo and in vitro - PubMed (original) (raw)
Stem cells from human exfoliated deciduous teeth correct the immune imbalance of allergic rhinitis via Treg cells in vivo and in vitro
Yu-Yang Dai et al. Stem Cell Res Ther. 2019.
Abstract
Background: Several studies have demonstrated that mesenchymal stem cells can ameliorate the inflammation of allergic rhinitis (AR) and correct the Th1/Th2 immune imbalance.
Methods: This study was performed to explore the immunomodulation properties of stem cells from human exfoliated deciduous teeth (SHEDs) in the treatment of AR in vivo and in vitro. BALB/c mice were sensitized to ovalbumin (OVA) by intraperitoneal injection, and then SHEDs or bone marrow mesenchymal stem cells (BMMSCs) were injected intravenously before challenge. We evaluated nasal symptoms, inflammatory infiltration of nasal mucosa, immunoglobulin secretion, cytokine production, and mRNA expression in the spleen. In addition, peripheral blood mononuclear cells (PBMCs) from AR patients were cultured with SHEDs or BMMSCs in the presence of phytohemagglutinin (PHA). PBMCs cultured alone with or without PHA served as controls. After 3 days of culture, we examined the effect of SHEDs on T lymphocyte proliferation, cytokine secretion, and the proportion of Foxp3+ Treg cells via flow cytometry. Finally, to determine the role of soluble factors (TGF-β1, PGE2) in the immunomodulatory mechanism, a cytokine neutralization assay was performed.
Results: Nasal symptoms and inflammatory infiltration were significantly reduced after SHED administration. The OVA-specific IgE and IgG1 levels in serum were significantly decreased, and the increased IL-4, IL-5, IL-13, and IL-17A levels in the spleen after OVA challenge were markedly downregulated, while the level of IFN-γ was upregulated by SHED administration. The mRNA expression levels also changed correspondingly. SHEDs significantly inhibited the proliferation of T lymphocytes; increased the levels of IFN-γ, IL-10, PGE2, and TGF-β1; decreased the levels of IL-4 and IL-17A; and induced the expansion of Treg cells in the coculture system. The neutralization of TGF-β1 partly relieved the immunosuppression of SHEDs, but blocking PGE2 did not. In addition, SHEDs were superior to BMMSCs in inhibiting the Th2 immune response in vivo and inducing the expansion of Treg cells in vitro.
Conclusion: These results suggest that SHEDs could correct the CD4+ T cell immune imbalance via Treg cells and may be potential therapeutic agents for the treatment of allergic diseases, such as AR, in the future.
Keywords: Allergic rhinitis; Immunoregulation; Regulatory T cells; Stem cells from human exfoliated deciduous teeth; Transforming growth factor beta.
Conflict of interest statement
Ethics approval and consent to participate
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Capital Medical University (AEEI-2018-075). The cell research was in compliance with the Helsinki declaration and was approved by the ethics committee of Beijing Tongren Hospital (TREC2018-KY01). The informed consent process was performed and informed consent form was signed by each patient.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
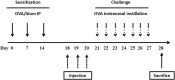
Fig. 1
The animal experimental protocol. Mice were sensitized on days 0, 7, and 14 by an intraperitoneal injection of OVA with aluminum hydroxide. From days 21 to 27, the mice were challenged with OVA intranasal instillation using a micropipette. Purified SHEDs or BMMSCs were injected via the tail vein on days 18, 19, and 20, and all mice were sacrificed on day 28
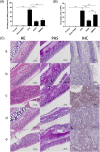
Fig. 2
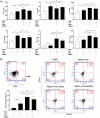
Effect of SHED on nasal inflammation. A The nasal symptoms were evaluated by the number of sneezing and rubbing events in 10 min after the final challenge. Nasal symptoms in the OVA group were much more serious than those in the control group and sham-SHED group but were relieved after SHED and BMMSC treatment. B, C Twenty-four hours after the last challenge, HE staining, PAS staining, and IHC were used to reflect the inflammatory infiltration in the nasal mucosa. The numbers of eosinophils were evaluated under a light microscope (× 400 magnification). Nearly no inflammatory cells were observed in the control group (a) and sham-SHED group (b). In contrast, the OVA group (c) exhibited obvious eosinophil infiltration, goblet cell hyperplasia, and T lymphocyte infiltration. The inflammatory infiltration in the SHED group (d) and BMMSC group (e) was significantly alleviated compared with the OVA group, and the eosinophil count in the SHED group was lower than that of the BMMSC group. Data are expressed as the mean ± SD (n = 6 in each group) from three representative experiments. ***p < .001
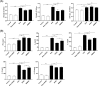
Fig. 3
Effect of SHED on antigen-specific antibody and cytokine production. OVA-specific IgE, IgG1, and IgG2a levels in serum (a) and characteristic cytokines of Th1, Th2, and Th17 cells in lymphocyte culture supernatant (b) were measured by ELISA kits. OVA-specific IgE, IgG1, and IgG2a levels were higher in the OVA group than in the control and sham-SHED groups. In addition, IgE and IgG1 were significantly reduced upon SHED and BMMSC treatments but not IgG2a. Both SHED and BMMSC treatments significantly enhanced the expression of IFN-γ but reduced IL-4, IL-5, IL-13, and IL-17A levels. The downregulation of IL-4 and IL-13 levels in the SHED group was more obvious compared with that in the BMMSC group at the same dose. Data are expressed as the mean ± SD (n = 6 in each group) from three representative experiments. *p < .05, **p < .01, ***p < .001
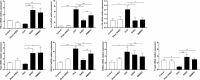
Fig. 4
Expression of mRNAs encoding cytokines and specific transcription factors. The relative mRNA expression levels of IL-4 and GATA-3 were significantly higher in the OVA group than in the control and sham-SHED groups, and such upregulation was inhibited by SHED and BMMSC administration. The IFN-γ, T-bet, and Foxp3 mRNA expression levels were markedly enhanced by SHED and BMMSC administration. In addition, MSCs reduced the mRNA expression of IL-17A, but RORγt expression in the SHED and BMMSC treatment groups had no significant change compared with that in the OVA group. The capacity of SHEDs in reducing IL-4 and GATA-3 levels was superior to that of BMMSCs. Data are expressed as the mean ± SD (n = 6 in each group) from three representative experiments. *p < .05, **p < .01, ***p < .001, n.s. no significance
Fig. 5
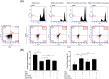
The immunosuppressive effect of SHEDs on PHA-stimulated lymphocyte proliferation. A CFSE-labeled PBMCs from AR patients (1 × 106/well) were stimulated by PHA (5 μg/ml) in a 24-well plate for 3 days in the presence or absence of different numbers of SHEDs or BMMSCs (n = 5). PBMCs were gated for flow cytometry analysis of CD3+ staining. B Representative results of T cell proliferation without stimulation (a), PHA (5 μg/ml) stimulation (b), and PHA stimulation in the presence of SHEDs (1 × 104/well, c), SHEDs (2 × 104/well, d), SHEDs (1 × 105/well, d), or BMMSCs (1 × 105/well, e) are shown. SHEDs significantly inhibited PHA-stimulated lymphocyte proliferation in a number-dependent manner, as determined by lymphocyte proliferation percentages. BMMSCs also inhibited T lymphocyte proliferation, but there was no significant difference with SHED at the same dose. The data are expressed as the mean ± SD. *p < .05, ***p < .001, n.s. no significance, compared with group on the left
Fig. 6
SHED corrected the immune imbalance and promoted the expansion of CD4+CD25+Foxp3+Treg cells in vitro. a The addition of SHEDs led to the downregulation of IL-4 and IL-17 levels and the upregulation of IFN-γ, IL-10, PGE2, and TGF-β1 levels. Although BMMSCs showed similar immunomodulation ability, the levels of IFN-γ and IL-4 were not significantly different from those of the PHA-stimulated PBMC (n = 10). b In addition, both SHEDs and BMMSCs upregulated the proportion of CD4+CD25+Foxp3+Treg cells in CD4+ subsets compared to the PHA-stimulated PBMC group, and the ratio of Treg cells in the SHED group was significantly higher than that in the BMMSC group (n = 5). The data are expressed as the mean ± SD. *p < .05, **p < 0.01, ***p < .001, n.s. no significance
Fig. 7
Results of the cytokine neutralization assay. a To reveal the role of TGF-β1 and PGE2 in the immunomodulatory mechanism of SHEDs, a cytokine neutralization assay was performed. Treg expansion was detected via flow cytometry, and T lymphocyte proliferation was evaluated by CFSE labeling (n = 5). b The percentage of T lymphocyte proliferation was significantly upregulated compared to that in the PBMC+PHA+SHED group upon anti-TGF-β Ab addition, and the percentage of Treg cells markedly decreased. In contrast, there was no significant change in the percentage of T lymphocyte proliferation and Treg cell frequency when indomethacin was added to block the PGE2 pathway. The data are expressed as the mean ± SD. *p < .05, ***p < .001, n.s. no significance
Similar articles
- Analysis of the expression changes of IL-17+ γδ T cells and Treg cells in bone marrow mesenchymal stem cells targeted therapy for allergic rhinitis.
Zou B, Zhuang RX, Sun XY, Liang J. Zou B, et al. Eur Rev Med Pharmacol Sci. 2021 Apr;25(7):2858-2865. doi: 10.26355/eurrev_202104_25539. Eur Rev Med Pharmacol Sci. 2021. PMID: 33877651 - Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β in Guinea Pigs.
Xu F, Yu S, Qin M, Mao Y, Jin L, Che N, Liu S, Ge R. Xu F, et al. Inflammation. 2018 Feb;41(1):81-92. doi: 10.1007/s10753-017-0666-6. Inflammation. 2018. PMID: 28894978 - [Research progress in therapeutic action of mesenchymal stem cell in allergic rhinitis].
Wei XM, Gao X, Yu CJ. Wei XM, et al. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018 Oct 7;53(10):789-793. doi: 10.3760/cma.j.issn.1673-0860.2018.10.015. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018. PMID: 30347542 Review. Chinese. - Effect of mesenchymal stem cell therapy in animal models of allergic rhinitis: A systematic review and meta-analysis.
Hong D, Hu Z, Weng J, Yang L, Xiong Y, Liu Y. Hong D, et al. Int Immunopharmacol. 2023 Nov;124(Pt B):111003. doi: 10.1016/j.intimp.2023.111003. Epub 2023 Oct 6. Int Immunopharmacol. 2023. PMID: 37806104 Review.
Cited by
- Sinking Our Teeth in Getting Dental Stem Cells to Clinics for Bone Regeneration.
Shoushrah SH, Transfeld JL, Tonk CH, Büchner D, Witzleben S, Sieber MA, Schulze M, Tobiasch E. Shoushrah SH, et al. Int J Mol Sci. 2021 Jun 15;22(12):6387. doi: 10.3390/ijms22126387. Int J Mol Sci. 2021. PMID: 34203719 Free PMC article. Review. - [Research advances of mesenchymal stem cell in allergic rhinitis].
Li W, Wang Y, Cheng F, Qi X, An Y, Zhao C. Li W, et al. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024 May;38(5):442-447;452. doi: 10.13201/j.issn.2096-7993.2024.05.018. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024. PMID: 38686485 Free PMC article. Review. Chinese. - Dental Follicle Cells: Roles in Development and Beyond.
Zhou T, Pan J, Wu P, Huang R, Du W, Zhou Y, Wan M, Fan Y, Xu X, Zhou X, Zheng L, Zhou X. Zhou T, et al. Stem Cells Int. 2019 Sep 15;2019:9159605. doi: 10.1155/2019/9159605. eCollection 2019. Stem Cells Int. 2019. PMID: 31636679 Free PMC article. Review. - Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance.
Negi N, Griffin MD. Negi N, et al. Stem Cells. 2020 May;38(5):596-605. doi: 10.1002/stem.3151. Epub 2020 Feb 3. Stem Cells. 2020. PMID: 31995249 Free PMC article. - Antibacterial and Immunomodulatory Properties of Stem Cells from Human Exfoliated Deciduous Teeth: An In Vitro Study.
Tyagi A, Shetty J, Shetty S, Kumar BM, Shetty AV, Nair MR. Tyagi A, et al. Int J Clin Pediatr Dent. 2023 Nov;16(Suppl 3):240-246. doi: 10.5005/jp-journals-10005-2683. Int J Clin Pediatr Dent. 2023. PMID: 38268633 Free PMC article.
References
- Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, Simmons AL, Wingertzahn MA, Boyle JM. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;12:443–470. - PubMed
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–1586. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials