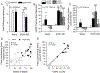Elevation of Transient Receptor Potential Vanilloid 1 Function in the Lateral Habenula Mediates Aversive Behaviors in Alcohol-withdrawn Rats - PubMed (original) (raw)
Elevation of Transient Receptor Potential Vanilloid 1 Function in the Lateral Habenula Mediates Aversive Behaviors in Alcohol-withdrawn Rats
Danielle M Gregor et al. Anesthesiology. 2019 Apr.
Abstract
What we already know about this topic: Chronic alcohol use and withdrawal leads to increased pain perception, anxiety, and depression. These aberrant behaviors are accompanied by increased excitatory glutamatergic transmission to, and activity of, the lateral habenula neurons.Vanilloid type 1, or TRPV1, channels are expressed in the habenula and they facilitate glutamatergic transmission. Whether TRPV1 channel plays a role in habenula hyperactivity is not clear.
What this article tells us that is new: Glutamatergic transmission in the lateral habenula was inhibited by TRPV1 channel antagonists. In vivo, local administration of TRPV1 antagonists into the lateral habenula attenuated hyperalgesia, anxiety, and relapse-like drinking in rats who chronically consumed alcohol.The data suggest that enhanced TRPV1 channel function during withdrawal may contribute to aberrant behavior that promotes relapse alcohol consumption.
Background: Recent rat studies indicate that alcohol withdrawal can trigger a negative emotional state including anxiety- and depression-like behaviors and hyperalgesia, as well as elevated glutamatergic transmission and activity in lateral habenula neurons. TRPV1, a vanilloid receptor expressed in the habenula, is involved in pain, alcohol dependence, and glutamatergic transmission. The authors therefore hypothesized that TRPV1 contributes to the changes in both the behavioral phenotypes and the habenula activity in alcohol-withdrawn rats.
Methods: Adult male Long-Evans rats (n = 110 and 280 for electrophysiology and behaviors, respectively), randomly assigned into the alcohol and water (Naïve) groups, were trained to consume either alcohol or water-only using an intermittent-access procedure. Slice electrophysiology was used to measure spontaneous excitatory postsynaptic currents and firing of lateral habenula neurons. The primary outcome was the change in alcohol-related behaviors and lateral habenula activity induced by pharmacologic manipulation of TRPV1 activity.
Results: The basal frequency of spontaneous excitatory postsynaptic currents and firing of lateral habenula neurons in alcohol-withdrawn rats was significantly increased. The TRPV1 antagonist capsazepine (10 µM) induced a stronger inhibition on spontaneous excitatory postsynaptic currents (mean ± SD; by 26.1 ± 27.9% [n = 11] vs. 6.7 ± 18.6% [n = 17], P = 0.027) and firing (by 23.4 ± 17.6% [n = 9] vs. 11.9 ± 16.3% [n = 12], P = 0.025) in Withdrawn rats than Naive rats. By contrast, the TRPV1 agonist capsaicin (3 μM) produced a weaker potentiation in Withdrawn than Naïve rats (spontaneous excitatory postsynaptic currents: by 203.6 ± 124.7% [n = 20] vs. 415.2 ± 424.3% [n = 15], P < 0.001; firing: 38.1 ± 14.7% [n = 11] vs. 73.9 ± 41.9% [n = 11], P < 0.001). Conversely, capsaicin's actions in Naïve but not in Withdrawn rats were significantly attenuated by the pretreatment of TRPV1 endogenous agonist N-Oleoyldopamine. In Withdrawn rats, intra-habenula infusion of TRPV1 antagonists attenuated hyperalgesia and anxiety-like behaviors, decreased alcohol consumption upon resuming drinking, and elicited a conditioned place preference.
Conclusions: Enhanced TRPV1 function may contribute to increased glutamatergic transmission and activity of lateral habenula neurons, resulting in the aberrant behaviors during ethanol withdrawal.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflicts of interest, financial or otherwise.
Figures
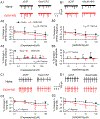
Fig. 1.. Enhanced glutamate transmission and activity of LHb neurons in ethanol-withdrawn rats.
(A) sEPSCs recorded from a slice of a Naïve rat (black) and a rat at 24hr withdrawal from chronic drinking in the IA2BC paradigm for two months (EtOH-WD, red). The events were eliminated by the glutamate receptor antagonists (50 μM AP5 and 20 µM DNQX). Cumulative probability plots of inter-event intervals (B1) and amplitudes of sEPSCs (C1). Summary data of frequency (B2) and amplitude (C2) of sEPSCs. (D) Spontaneous firing of LHb neurons recorded in the cell-attached mode in the absence and presence of AP5 plus DNQX in Naïve and EtOH-WD rats. (E) Firing rate of LHb neurons from Naïve and EtOH-WD rats. (F1) Firing rate of LHb neurons of Naïve and EtOH-WD rats before and after the application of AP5+DNQX. (F2) Inhibition of firing by AP5+DNQX was significantly greater in EtOH-WD rats than in Naïve rats. Data are expressed as mean ± SD. #P < 0.05, ##P < 0.01 EtOH-WD compared with the naïve group, student unpaired _t_-test (two-tailed). &P < 0.05, &&&P < 0.001, aCSF compared with AP5+DNQX, two-way repeated-measure ANOVA followed by Tukey post hoc comparison. Cell numbers in each figure are indicated.
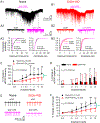
Fig.2.. TRPV1 antagonist capsazepine and AMG9810 induce a stronger inhibition of glutamate transmission and firing in LHb neurons from EtOH-WD rats.
Representative traces of sEPSCs in the absence and presence of capsazepine (CPZ, A1) and AMG9810 (AMG, B1) in Naïve (●) and EtOH-WD (△) rats. Capsazepine and AMG9810 (0.1–100 μM) induced changes in the frequency (CPZ: A2, AMG: B2) and amplitude (CPZ: A3, AMG: B3) of sEPSCs. Example traces of firing in the absence and presence of capsazepine (C1) and AMG9810 (D1). Summary graph of inhibition induced by capsazepine (C2) and AMG9810 (D2). Capsazepine and AMG9810 produced a significantly greater inhibition on sEPSC frequency and firing rate in EtOH-WD rats. *P < 0.05, **P < 0.01, ***P < 0.001 relative to 0.1 μM CPZ/AMG. #P < 0.05, ##P < 0.01 Naïve in comparison with EtOH-WD rats. Data were analyzed with two-way ANOVA and Tukey post hoc comparison.
Fig.3.. TRPV1 agonist capsaicin induces a stronger potentiation of glutamate transmission and firing in LHb neurons from ethanol naive rats.
Representative traces showing enhancement of sEPSCs induced by 3 µM capsaicin in LHb neurons from a Naive (A1) or an EtOH-WD (B1) rat. (A2-B2) Exemplar current traces were acquired in A1 and B1, before and during capsaicin application. Cumulative probability plots show higher incidence of events with shorter inter-event interval, and amplitude before and after capsaicin application in the LHb neurons of Naive (A3) and EtOH-WD (B3) rats (k-s test). Capsaicin elicited a concentration-dependent increase in sEPSC frequency (C), which was significantly greater in Naïve than in EtOH-WD neurons. The smooth curve is the best fit to the data by the logistic equation. Capsaicin did not significantly alter the mean sEPSC amplitude (D). (E) A representative example of increased spontaneous firing rate induced by 3 µM capsaicin in a Naïve or an EtOH-WD neuron. (F) Capsaicin caused a concentration-dependent increase in firing rate, which was significantly greater in the Naïve than the EtOH-WD neurons. *P < 0.05, **P < 0.01, ***P < 0.001 relative to 0.01 μM capsaicin; ###P < 0.001, Naïve in comparison with EtOH-WD rats. ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, Naïve in comparison with EtOH-WD rats underwent the same dose of capsaicin. Data were analyzed with two-way ANOVA and Tukey post hoc comparison.
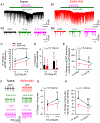
Fig.4.. Pretreatment with endogenous TRPV1 agonist N-oleoyldopamine attenuates capsaicin-induced enhancement in glutamate transmission and firing in LHb neurons.
Representative traces showing enhancement of sEPSCs induced by N-oleoyldopamine (OLDA,10 μM) followed by capsaicin (3 μM) in a Naive (A1) and an EtOH-WD (B1) neuron. (A2-B2) Exemplar current traces were acquired in A1 and B1, before and during OLDA application, and OLDA/capsaicin co-application. Application of a high concentration OLDA (10 μM) enhanced sEPSC frequency (C) in LHb neurons of naïve but not in EtOH-WD rats. (D) OLDA did not alter sEPSC amplitude. (E) In the presence of 10 μM OLDA, capsaicin (3μM) produced a weaker increase on sEPSC frequency in naïve rats. (F) Representative traces showing enhancement of firing induced by OLDA alone or OLDA/capsaicin co-application in neurons of Naïve and EtOH-WD rats. (G) OLDA (10 μM) activated the LHb neurons of naïve rats but not that of EtOH-WD rats. (H) With the pretreatment of OLDA (10 μM), capsaicin (3 μM) produced a weaker increase in firing in naïve rats. *P < 0.05, **P < 0.01 relative to respective 0.2μM OLDA. ##P < 0.01, Naïve in comparison with EtOH-WD rats. @P < 0.05, @@P < 0.01, capsaicin-induced change within OLDA comparison to without OLDA (aCSF).
Fig. 5.. Capsazepine mitigates hyperalgesia and spontaneous pain in ethanol-withdrawn rats.
(A) The paw withdrawal latency (PWL) to thermal stimuli was significantly reduced in EtOH-WD rats at 24hr withdrawal, in comparison to the Naïve counterparts. $P < 0.001 relative to respective baseline. Two-way RM ANOVA followed by Tukey post hoc comparison. (B) Intra-LHb injection of capsazepine (CPZ) significantly increased the nociceptive response in EtOH-WD rats. The change in nociceptive response is expressed as percent maximum peak effect (%MPE). Intra-LHb DNQX significantly increased PWL in both Naïve and EtOH-WD rats. (C) In the place conditioning paradigm, intra-LHb CPZ significantly increased the place conditioning score in EtOH-WD rats; intra-LHb DNQX significantly increased the CPP score in both Naïve and EtOH-WD rats. **P <0.01, ***P < 0.001 relative to respective aCSF treatment; ###P < 0.001 Naïve vs. EtOH-WD rats. The place conditioning score was significantly correlated with %MPE with intra-LHb DNQX (D), and CPZ (E) in EtOH-WD rats.
Fig. 6.. Activation of LHb TRPV1 channels contributes to anxiety- and depression-like behaviors.
(A-D) Elevated Plus Maze (EPM) Data. (A1, A4) Representative traces show EtOH-WD rats spend less time in open arms compared to Naive rats. In Naïve rats, intra-LHb either capsazepine (CPZ, A2) or capsaicin (CAPS, A3), did not alter the time in (B), and the entries (C) to the open arms. In EtOH-WD rats, CPZ (A5) or CAPS (A6) significantly increased open arm time (B). (D) The total distance traveled in the EPM was not changed. (E) In the marble burying test, EtOH-WD rats buried significantly more marbles than Naïve rats. In Naïve rats, CAPS increased the marbles buried; in EtOH-WD rats, CPZ or CAPS significantly decreased the marbles buried. (F, G), In the forced swimming test, EtOH-WD rats had a significantly shorter latency to first immobility (F) and longer total immobility time (G) compared to naïve rats. CPZ did not alter the latency or total immobility time, while CAPS significantly reduced the latency and increase the immobility time in Naïve rats. *P<0.05, **P<0.01, ***P<0.001 relative to respective aCSF. ##P < 0.01, ###P < 0.001, Naïve in comparison with EtOH-WD rats. Data were analyzed with two-way ANOVA and Tukey post hoc comparison.
Fig.7.. Intra-LHb capsazepine and capsaicin significantly decreases ethanol intake (A), preference (B), and total fluid intake (D), but increases water intake (C).
**P < 0.01, ***P < 0.001 relative to respective aCSF treatment, two-way ANOVA followed by Tukey post hoc test.
Fig. 8.. Cartoon demonstrating that repeated alcohol and withdrawal can increase endogenous TRPV1 activity which may increase glutamate (Glu) release and hyperactivity of lateral habenula (LHb) neurons and hyperalgesia and alcohol intake.
Similar articles
- Adaptation in 5-HT2 receptors-CaMKII signaling in lateral habenula underlies increased nociceptive-sensitivity in ethanol-withdrawn rats.
Zuo W, Wu L, Mei Q, Zuo Q, Zhou Z, Fu R, Li W, Wu W, Matthew L, Ye JH. Zuo W, et al. Neuropharmacology. 2019 Nov 1;158:107747. doi: 10.1016/j.neuropharm.2019.107747. Epub 2019 Aug 22. Neuropharmacology. 2019. PMID: 31445991 - Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats.
Kang S, Li J, Bekker A, Ye JH. Kang S, et al. Neuropharmacology. 2018 Feb;129:47-56. doi: 10.1016/j.neuropharm.2017.11.013. Epub 2017 Nov 8. Neuropharmacology. 2018. PMID: 29128307 Free PMC article. - Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula.
Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH. Kang S, et al. Neuropsychopharmacology. 2017 Aug;42(9):1813-1824. doi: 10.1038/npp.2017.68. Epub 2017 Apr 7. Neuropsychopharmacology. 2017. PMID: 28387223 Free PMC article. - The lateral habenula and alcohol: Role of glutamate and M-type potassium channels.
Shah A, Zuo W, Kang S, Li J, Fu R, Zhang H, Bekker A, Ye JH. Shah A, et al. Pharmacol Biochem Behav. 2017 Nov;162:94-102. doi: 10.1016/j.pbb.2017.06.005. Epub 2017 Jun 15. Pharmacol Biochem Behav. 2017. PMID: 28624587 Review. - Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models.
Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Chen G, et al. Alcohol Clin Exp Res. 2011 Oct;35(10):1739-48. doi: 10.1111/j.1530-0277.2011.01520.x. Epub 2011 May 25. Alcohol Clin Exp Res. 2011. PMID: 21615425 Free PMC article. Review.
Cited by
- Activation of glycine receptors in the lateral habenula rescues anxiety- and depression-like behaviors associated with alcohol withdrawal and reduces alcohol intake in rats.
Li W, Zuo W, Wu W, Zuo QK, Fu R, Wu L, Zhang H, Ndukwe M, Ye JH. Li W, et al. Neuropharmacology. 2019 Oct;157:107688. doi: 10.1016/j.neuropharm.2019.107688. Epub 2019 Jun 27. Neuropharmacology. 2019. PMID: 31254534 Free PMC article. - The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review.
Catalfamo LM, Marrone G, Basilicata M, Vivarini I, Paolino V, Della-Morte D, De Ponte FS, Di Daniele F, Quattrone D, De Rinaldis D, Bollero P, Di Daniele N, Noce A. Catalfamo LM, et al. Int J Environ Res Public Health. 2022 Sep 6;19(18):11187. doi: 10.3390/ijerph191811187. Int J Environ Res Public Health. 2022. PMID: 36141454 Free PMC article. Review. - The intersection of astrocytes and the endocannabinoid system in the lateral habenula: on the fast-track to novel rapid-acting antidepressants.
Arjmand S, Landau AM, Varastehmoradi B, Andreatini R, Joca S, Wegener G. Arjmand S, et al. Mol Psychiatry. 2022 Aug;27(8):3138-3149. doi: 10.1038/s41380-022-01598-4. Epub 2022 May 18. Mol Psychiatry. 2022. PMID: 35585261 Review. - Endocannabinoid signaling in the lateral habenula regulates pain and alcohol consumption.
Fu R, Tang Y, Li W, Ren Z, Li D, Zheng J, Zuo W, Chen X, Zuo QK, Tam KL, Zou Y, Bachmann T, Bekker A, Ye JH. Fu R, et al. Transl Psychiatry. 2021 Apr 14;11(1):220. doi: 10.1038/s41398-021-01337-3. Transl Psychiatry. 2021. PMID: 33854035 Free PMC article. - Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence.
Li J, Chen P, Han X, Zuo W, Mei Q, Bian EY, Umeugo J, Ye J. Li J, et al. Int J Physiol Pathophysiol Pharmacol. 2019 Aug 25;11(4):163-176. eCollection 2019. Int J Physiol Pathophysiol Pharmacol. 2019. PMID: 31523363 Free PMC article.
References
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA: Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One 2014; 9: e92701. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical