Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates - PubMed (original) (raw)
Review
Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates
Simon Alberti et al. Cell. 2019.
Abstract
Evidence is now mounting that liquid-liquid phase separation (LLPS) underlies the formation of membraneless compartments in cells. This realization has motivated major efforts to delineate the function of such biomolecular condensates in normal cells and their roles in contexts ranging from development to age-related disease. There is great interest in understanding the underlying biophysical principles and the specific properties of biological condensates with the goal of bringing insights into a wide range of biological processes and systems. The explosion of physiological and pathological contexts involving LLPS requires clear standards for their study. Here, we propose guidelines for rigorous experimental characterization of LLPS processes in vitro and in cells, discuss the caveats of common experimental approaches, and point out experimental and theoretical gaps in the field.
Copyright © 2018. Published by Elsevier Inc.
Figures
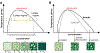
Figure 1:. Schematic phase diagram.
The coexistence line (black) separates the one-phase and two-phase regime and is a function of environmental conditions such as temperature, pH etc. The system does not undergo phase separation beyond the critical point. (A) At concentrations below csat, the system is in the one-phase regime. At any condition within the two-phase regime, the system demixes into a light phase (with c=cL) and a dense phase (with c=cD). All conditions on a single tie line (the orange line is an example) result in two phase systems with fixed light phase and dense phase concentrations, cL and cD, respectively; only the volume fractions of the two phases, fL and fD, change relatively to each other (examples 2 – 4). The volume fractions resulting from demixing of condition 3 can be calculated by the lever rule and are fL = D/T (i.e. the ratio of the lengths of D and T) for the light phase and fD = L/T for the dense phase. In equilibrium, csat and cL are equivalent, but when phase separation is nucleated, csat and cL can differ during the ripening dynamics of the system. (B) The spinodal (grey line) indicates the region of instability in which the system must undergo demixing via spinodal decomposition. In the area between the coexistence line, or binodal, and the spinodal, the system demixes when nucleated.
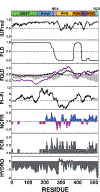
Figure 2:. Bioinformatic analysis of the amino acid sequence of FUS to identify protein regions that are involved in phase separation.
Schematic representation of the human FUS/TLS domain structure is shown on top. QGSY: region enriched for the residues glutamine (Q), glycine (G), serine (S) and tyrosine (Y) depicted in green, G-rich: region enriched for glycine residues depicted in blue, NES: nuclear export sequence depicted in magenta, RRM: RNA recognition motif depicted in yellow, RGG: region enriched for residues arginine and glycine depicted in orange, ZN: Zinc finger domain depicted in purple, NLS: nuclear localization sequence depicted light blue. IUPred: Prediction of intrinsic disorder, PLD: Prediction of prion-like region (PLAAC), FOLD: Intrinsic disorder prediction by PLAAC (black) and PAPA (purple). Fold index is shown in gray. Pi-Pi: Pi interaction prediction NCPR: Net charge per residue (sliding window size of 10), FCR: Fraction of charged residues, HYDRO: hydrophobicity (Kyte & Doolittle, sliding Windows size of 9).
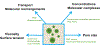
Figure 3:
Overview of experimental approaches used to evaluate properties of assemblies formed by LLPS
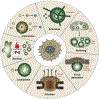
Figure 4:
An initial functional repertoire of biomolecular condensates.
Similar articles
- Protein phase separation and its role in chromatin organization and diseases.
Li J, Zhang Y, Chen X, Ma L, Li P, Yu H. Li J, et al. Biomed Pharmacother. 2021 Jun;138:111520. doi: 10.1016/j.biopha.2021.111520. Epub 2021 Mar 23. Biomed Pharmacother. 2021. PMID: 33765580 Review. - Formation of biological condensates via phase separation: Characteristics, analytical methods, and physiological implications.
Feng Z, Chen X, Wu X, Zhang M. Feng Z, et al. J Biol Chem. 2019 Oct 4;294(40):14823-14835. doi: 10.1074/jbc.REV119.007895. Epub 2019 Aug 23. J Biol Chem. 2019. PMID: 31444270 Free PMC article. Review. - In Vitro Liquid-Liquid Phase Separation Assay for Viral Proteins.
Hu M, Liu X, Liang T, Zheng C, Ma X. Hu M, et al. Methods Mol Biol. 2025;2940:63-78. doi: 10.1007/978-1-0716-4615-1_7. Methods Mol Biol. 2025. PMID: 40515902 - Phase separation in RNA biology.
Lin Y, Fang X. Lin Y, et al. J Genet Genomics. 2021 Oct 20;48(10):872-880. doi: 10.1016/j.jgg.2021.07.012. Epub 2021 Aug 8. J Genet Genomics. 2021. PMID: 34371110 Review. - Protein Databases Related to Liquid-Liquid Phase Separation.
Li Q, Wang X, Dou Z, Yang W, Huang B, Lou J, Zhang Z. Li Q, et al. Int J Mol Sci. 2020 Sep 16;21(18):6796. doi: 10.3390/ijms21186796. Int J Mol Sci. 2020. PMID: 32947964 Free PMC article. Review.
Cited by
- Learning the molecular grammar of protein condensates from sequence determinants and embeddings.
Saar KL, Morgunov AS, Qi R, Arter WE, Krainer G, Lee AA, Knowles TPJ. Saar KL, et al. Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2019053118. doi: 10.1073/pnas.2019053118. Proc Natl Acad Sci U S A. 2021. PMID: 33827920 Free PMC article. - Condensation of the fusion focus by the intrinsically disordered region of the formin Fus1 is essential for cell-cell fusion.
Billault-Chaumartin I, Muriel O, Michon L, Martin SG. Billault-Chaumartin I, et al. Curr Biol. 2022 Nov 7;32(21):4752-4761.e10. doi: 10.1016/j.cub.2022.09.026. Epub 2022 Oct 5. Curr Biol. 2022. PMID: 36202103 Free PMC article. - Poly(ADP-ribose) in Condensates: The PARtnership of Phase Separation and Site-Specific Interactions.
Alemasova EE, Lavrik OI. Alemasova EE, et al. Int J Mol Sci. 2022 Nov 15;23(22):14075. doi: 10.3390/ijms232214075. Int J Mol Sci. 2022. PMID: 36430551 Free PMC article. Review. - ArcRNAs and the formation of nuclear bodies.
Nakagawa S, Yamazaki T, Mannen T, Hirose T. Nakagawa S, et al. Mamm Genome. 2022 Jun;33(2):382-401. doi: 10.1007/s00335-021-09881-5. Epub 2021 Jun 3. Mamm Genome. 2022. PMID: 34085114 Review. - Short PolyA RNA Homopolymers Undergo Mg2+-Mediated Kinetically Arrested Condensation.
Tom JKA, Onuchic PL, Deniz AA. Tom JKA, et al. J Phys Chem B. 2022 Nov 24;126(46):9715-9725. doi: 10.1021/acs.jpcb.2c05935. Epub 2022 Nov 15. J Phys Chem B. 2022. PMID: 36378781 Free PMC article.
References
- Alberti S (2017). The wisdom of crowds: regulating cell function through condensed states of living matter. J. Cell Sci 130, jcs.200295. - PubMed
- Alberti S, and Carra S (2018). Quality Control of Membraneless Organelles. J. Mol. Biol - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources