Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice - PubMed (original) (raw)
Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice
Haidong Wang et al. Mol Ther. 2019.
Abstract
Our previous study showed that miR-29 attenuates muscle wasting in chronic kidney disease. Other studies found that miR-29 has anti-fibrosis activity. We hypothesized that intramuscular injection of exosome-encapsulated miR-29 would counteract unilateral ureteral obstruction (UUO)-induced muscle wasting and renal fibrosis. We used an engineered exosome vector, which contains an exosomal membrane protein gene Lamp2b that was fused with the targeting peptide RVG (rabies viral glycoprotein peptide). RVG directs exosomes to organs that express the acetylcholine receptor, such as kidney. The intervention of Exo/miR29 increased muscle cross-sectional area and decreased UUO-induced upregulation of TRIM63/MuRF1 and FBXO32/atrogin-1. Interestingly, renal fibrosis was partially depressed in the UUO mice with intramuscular injection of Exo/miR29. This was confirmed by decreased TGF-β, alpha-smooth muscle actin, fibronectin, and collagen 1A1 in the kidney of UUO mice. When we used fluorescently labeled Exo/miR29 to trace the Exo/miR route in vivo and found that fluorescence was visible in un-injected muscle and in kidneys. We found that miR-29 directly inhibits YY1 and TGF-β3, which provided a possible mechanism for inhibition of muscle atrophy and renal fibrosis by Exo/miR29. We conclude that Exo/miR29 ameliorates skeletal muscle atrophy and attenuates kidney fibrosis by downregulating YY1 and TGF-β pathway proteins.
Keywords: FBXO32/atrogin-1; TGF-β1; TGF-β3; TRIM63/MuRF1; Yin Yang 1; α-SMA.
Copyright © 2019 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
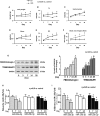
Figure 1
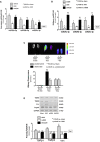
Evidence for Muscle Atrophy in UUO Mice (A–E) The graphs are body weight (A) and the ratio of skeletal muscle mass to body weight at 3, 7, 14, and 28 days after UUO surgery: (B) tibialis anterior (TA), (C) gastrocnemius, (D) soleus, and (E) extensor digitorum longus (EDL). (F) Blood urea nitrogen measured at different time periods using BLOOD UREA NITROGEN Kinetic Procedure Kit in plasma of control and UUO mice. Results are mean ± SE; n = 9/group; #p < 0.05 versus shams. (G) The two E3 ubiquitin ligase proteins TRIM63/MuRF1 and FBXO32/atrogin-1 were measured in the TA of sham and UUO mice at 0, 3, 7, 14, and 28 days after UUO surgery. Left: representative western blot of the marker proteins. Right: bar graphs showing the fold change of each protein band compared with levels in sham control mice (represented by 1-fold). All protein band densities have been normalized to the appropriate GAPDH loading control (bars: mean ± SE; n = 4/group; #p < 0.05 versus control). (H and I) Total RNA was extracted from skeletal muscle (H) and kidney (I) of sham (day 0) and UUO mice. The expressions of miR-29a-3p, miR-29b-3p, and miR-29c-3p were assayed by real-time qPCR at 0, 7, and 14 days after UUO surgery. The bar graph shows microRNA from the TA and kidney of each group of mice compared with levels in controls (represented by 1-fold). Results are normalized to U6 (bars: mean ± SE; n = 9/group; #p < 0.05 versus control).
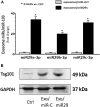
Figure 2
Exo/miR-29 Was Generated from Cultured Cells (A) The expression of miR-29 in the exosomes isolated from medium. The Lamp2b-RVG vector was transfected into satellite cells using Effectene (transfection reagent; QIAGEN, Valencia, CA, USA). After 6 h of transfection, the cells were transduced with Ad-miR29abc (adenovirus containing miR-29ab1 and miR-29b2c) or Ad-empty (control virus) in extracellular vesicle free serum (EVFS)-containing medium. Fresh EVFS medium was changed after 24 h. All cells were cultured for an additional 48 h to allow exosome release into the medium. The exosomes that are enriched with miR29abc (exosome/miR29) or control (exosome/miR-ctrl) were harvested from culture medium. RNA was isolated from exosomes. The expressions of miR-29a-3p, miR-29b-3p, and miR-29c-3p were assayed by real-time qPCR. The bar graph shows miR expression from the Exo/ctrl (represented by 1-fold) compared with levels in Exo/miR-29s. Results are normalized to miR103a (bars: mean ± SE; n = 6/group; *p < 0.05 versus Exo/ctrl). (B) Exosomes were isolated from the satellite cell culture medium. The protein from cell lysis was used as control. The exosome marker protein, Tsg101, was assayed by western blotting.
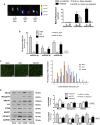
Figure 3
Exo/miR-29 Attenuated UUO-Induced Muscle Loss Mice were injected with exosomes carrying either miR-control or miR-29. (A) Mice were injected in the left TA with Exo/miR29 labeled with 1 μmol/L fluorescent lipophilic tracer DiR at the same time as UUO surgery. The injection was repeated once per week. The fluorescence was assessed in muscle at 14 days after injection. Panels from left to right: sham operated with no exosome injection, UUO mouse at 14 days (twice injection) after DiR∼Exo/miR29 injection, and sham operated with DiR∼exosome injection. In each pair, the left TA received the Exo/miR29 injection and the right did not. Fluorescent images were acquired using a Bruker Small Animal Optical Imaging System (In-Vivo Xtreme II). The white color indicates fluorescence level over maximal measurement limits. The picture with all organs compared is shown in Figure S1. The bar graph compares fluorescence intensity from each muscle (bars: mean ± SE; n = 3/group; *p < 0.05 versus sham injected muscle; #p < 0.05 versus sham non-injected muscle). (B) Total RNA was isolated from TA of sham plus Exo/miR-ctrl (sham), sham plus Exo/miR29 (miR29), UUO plus Exo/miR-ctrl (UUO), and UUO plus Exo/miR-29 mice (UUO/29). The expressions of miR-29a-3p, miR-29b-3p, and miR-29c-3p were assayed by real-time qPCR. The bar graph shows expression levels of the three miRs in each treatment group compared with levels in sham mice (represented by a line at 1-fold). Results are normalized to U6 (bars: mean ± SE; n = 9/group; *p < 0.05 versus sham; #p < 0.05 versus UUO). (C) The representative cross-sectional area of TA of sham plus Exo/miR-ctrl (sham), UUO plus Exo/miR-ctrl (UUO), and UUO plus Exo/miR-29 mice (UUO/29). Cryosections of TA were immunostained with anti-laminin antibody. The bar graph shows the frequency distribution of fiber cross-sectional areas in sham (blue), UUO (orange), and UUO/29 (green) mice (data are mean ± SE; n = 6/group; *p < 0.05 versus sham; #p < 0.05 versus UUO). (D) Shown are representative western blots of the muscle regeneration- and atrophy-related proteins myoD, myogenin, eMyHC, YY1, PTEN, MuRF1, and atrogin1 in muscle lysates from the different treatment groups of mice. All blots were also probed for GAPDH, and all protein band densities have been normalized to their corresponding GAPDH loading control. The bar graph shows the fold change of each protein band compared with levels in sham mice (represented by a line at 1-fold) (bars: mean ± SE; n = 9/group; *p < 0.05 versus sham; #p < 0.05 versus UUO).
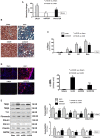
Figure 4
Intramuscular Injection of Exo/miR29 Attenuated Renal Fibrosis in the Kidney of UUO Mice Mice were injected with exosomes carrying either miR-control or miR-29. (A) Blood urea nitrogen was measured in sham plus Exo/miR-ctrl (sham), sham plus Exo/miR29 (miR29), UUO plus Exo/miR-ctrl (UUO), and UUO plus Exo/miR-29 mice (UUO/29). The bar graph shows the BLOOD UREA NITROGEN levels in each group compared with levels in sham mice (represented by a line) (bars: mean ± SE; n = 9/group; *p < 0.05 versus sham; #p < 0.05 versus UUO). (B) Shown is representative Masson’s trichrome staining of paraffin sections from kidneys of the four treatment groups. (C) The bar graph shows collagen amount in Masson’s trichrome staining measured at days 3, 5, 7, and 14. Results show the fold change compared with sham levels represented by a line at 1-fold (bars: mean ± SE; n = 6/group; *p < 0.05 versus control). (D) Representative kidney cryosections in each group were immunostained with anti-alpha smooth muscle actin (α-SMA) antibody. The bar graph shows α-SMA amount measured at day 7. Results show the fold change compared with sham levels defined as 1-fold (bars: mean ± SE; n = 6/group; *p < 0.05 versus control). (E) Shown are representative western blots of fibrosis-related proteins, TGF-β1, TGFβ-3, fibronectin, vimentin, collagen 1α1, collagen 4α1, and YY1, in kidney lysates from different groups of mice. The bar graph shows the fold change of the protein band compared with levels in sham mice represented by a line at 1-fold. All blots were also probed for GAPDH, and all protein band densities have been normalized to their corresponding GAPDH loading control (bars: mean ± SE; n = 9/group; *p < 0.05 versus sham; #p < 0.05 versus UUO).
Figure 5
Evidence of Exo/miR-29 in Kidney Mice were injected with exosomes carrying either miR-control or miR-29. (A and B) Total RNA was isolated from (A) serum exosomes and (B) whole kidney of sham plus Exo/miR-ctrl (sham), sham plus Exo/miR29 (miR29), UUO plus Exo/miR-ctrl (UUO), and UUO plus Exo/miR-29 mice (UUO/29). The expressions of miR-29a-3p, miR-29b-3p, and miR-29c-3p were assayed by real-time qPCR. The bar graphs show miR expression of each group of mice compared with levels in control mice (represented by a line at 1-fold). (A) Bars represent mean ± SE; n = 6/group. *p < 0.05 versus sham; #p < 0.05 versus UUO. Results are normalized to miR103a. (B) Bars represent mean ± SE; n = 6/group. *p < 0.05 versus sham; #p < 0.05 versus UUO; &p < 0.05 versus miR29. Results are normalized to U6. (C) Mice were injected in the left TA with Exo/miR-29 labeled with 1 μmol/L fluorescent lipophilic tracer DiR at the same time as UUO surgery. The injection was repeated once per week. Shown are representative fluorescent kidney images at 14 days that were acquired using a Bruker Small Animal Optical Imaging System. Panels from left to right: sham operated with no exosome injection, UUO mouse with exosome injection, and sham-operated mouse with exosome injection. The bar graph shows fluorescence intensities of the kidneys. The original whole picture was shown in Figure S1 (bars: mean ± SE; n = 3/group; *p < 0.05, UUO versus sham mouse; #p < 0.05, UUO mouse obstructed kidney versus UUO mouse unobstructed kidney). (D) Circulation exosomes from different group mice were solubilized in Laemmli sample buffer, and fibrosis-related proteins TGF-β1, TGF-β3, and YY1 were analyzed by western blot. All blots were also probed for GAPDH, and all protein band densities have been normalized to their corresponding GAPDH loading control. The bar graph shows the fold change of each protein band compared with levels in control plus Exo/miR-ctrl (represented by a line at 1-fold) (bars: mean ± SE; n = 9/group; *p < 0.05 versus UUO; #p < 0.05 versus sham).
Figure 6
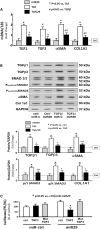
TGF-β3 Is Directly Targeted by miR-29 (A) Cultured HEK293 cells were treated with Ad-miR-ctrl (ctrl), Ad-miR29 (miR29), Ad-miR-ctrl + 10 ng/mL TGF-β (TGF-β), and Ad-miR-29 + TGF-β (TGF-β/29). Total RNA was extracted from the cells 24 h after treatment. The expressions of TGF-β1, TGF-β3, α-SMA, and collagen 1A1 (COL1A1) were assayed by real-time qPCR. The bar graph shows mRNA from the cells of each group compared with levels in Ad-miR-ctrl (represented by a line as 1-fold). Results are normalized to 18S (bars: mean ± SE; n = 9/group; *p < 0.05 versus Exo/miR-ctrl; #p < 0.05 versus Exo/miR-ctrl + TGF-β). (B) The fibrosis-related proteins TGF-β1, TGF-β3, SMAD 2/3, and phosphorylated SMAD 2/3 (pSer465 or pSer423 SMAD2/3) and collagen 1A1 were measured by western blotting in HEK293 cell lysates following the treatments described in (A). The bar graph shows total proteins or the ratios of each phospho-SMAD to total SMAD protein (p/t). All blots were also probed for GAPDH, and all protein band densities have been normalized to their corresponding GAPDH loading control. The fold change of the protein band or ratio is compared with levels in control plus Exo/miR-ctrl (represented by a line at 1-fold) (bars: mean ± SE; n = 9/group; *p < 0.05 versus UUO; #p < 0.05 versus sham). (C) Luciferase assessment of miR-29 binding on 3′ UTR of TGF-β3. HEK293 cells were transfected with luciferase control plasmid (pLuc-ctrl), a plasmid containing the 3′ UTR of TGF-β3 (pMIR-TGF-β3/838-844), and a plasmid containing mutated 3′ UTR of TGF-β3 (pMIR-mut-TGF-β3). Cells were co-transfected with renilla luciferase. After 6 h, cells were transduced with adenovirus containing miR29 (miR29) or control virus (miR-ctrl), and luciferase activity was assayed after an additional 24 h. Luciferase activity in cells that received the pLuc-ctrl with miR-ctrl was designated as 100%. The other bars show the response to miR-29 expressed as a percent of the control. Triplicate determinations were made in each condition, and each experiment was repeated a total of three times; the firefly luciferase (FFL) results were normalized by renilla luciferase (RL) activity. Data are mean ± SE; n = 9. *p < 0.05 versus pLuc+Ad-ctrl.
Similar articles
- Exogenous miR-29a Attenuates Muscle Atrophy and Kidney Fibrosis in Unilateral Ureteral Obstruction Mice.
Wang B, Wang J, He W, Zhao Y, Zhang A, Liu Y, Hassounah F, Ma F, Klein JD, Wang XH, Wang H. Wang B, et al. Hum Gene Ther. 2020 Mar;31(5-6):367-375. doi: 10.1089/hum.2019.287. Epub 2020 Feb 21. Hum Gene Ther. 2020. PMID: 31950871 Free PMC article. - miR-26a Limits Muscle Wasting and Cardiac Fibrosis through Exosome-Mediated microRNA Transfer in Chronic Kidney Disease.
Wang B, Zhang A, Wang H, Klein JD, Tan L, Wang ZM, Du J, Naqvi N, Liu BC, Wang XH. Wang B, et al. Theranostics. 2019 Mar 7;9(7):1864-1877. doi: 10.7150/thno.29579. eCollection 2019. Theranostics. 2019. PMID: 31037144 Free PMC article. - Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease.
Zhang A, Wang H, Wang B, Yuan Y, Klein JD, Wang XH. Zhang A, et al. FASEB J. 2019 Dec;33(12):13590-13601. doi: 10.1096/fj.201900884R. Epub 2019 Oct 8. FASEB J. 2019. PMID: 31593640 Free PMC article. - MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases.
He Y, Huang C, Lin X, Li J. He Y, et al. Biochimie. 2013 Jul;95(7):1355-9. doi: 10.1016/j.biochi.2013.03.010. Epub 2013 Mar 28. Biochimie. 2013. PMID: 23542596 Review. - Role of exosomes and exosomal microRNA in muscle-Kidney crosstalk in chronic kidney disease.
Zhou S, Cheing GLY, Cheung AKK. Zhou S, et al. Front Cell Dev Biol. 2022 Sep 7;10:951837. doi: 10.3389/fcell.2022.951837. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158193 Free PMC article. Review.
Cited by
- Engineered Extracellular Vesicles: A potential treatment for regeneration.
Cheng W, Xu C, Su Y, Shen Y, Yang Q, Zhao Y, Zhao Y, Liu Y. Cheng W, et al. iScience. 2023 Oct 24;26(11):108282. doi: 10.1016/j.isci.2023.108282. eCollection 2023 Nov 17. iScience. 2023. PMID: 38026170 Free PMC article. Review. - Bibliometric analysis of scientific papers on extracellular vesicles in kidney disease published between 1999 and 2022.
Hun M, Wen H, Han P, Vun T, Zhao M, He Q. Hun M, et al. Front Cell Dev Biol. 2023 Jan 5;10:1070516. doi: 10.3389/fcell.2022.1070516. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684427 Free PMC article. - Exosome-Based Drug Delivery: Translation from Bench to Clinic.
Koh HB, Kim HJ, Kang SW, Yoo TH. Koh HB, et al. Pharmaceutics. 2023 Jul 29;15(8):2042. doi: 10.3390/pharmaceutics15082042. Pharmaceutics. 2023. PMID: 37631256 Free PMC article. Review. - Exogenous miR-29a Attenuates Muscle Atrophy and Kidney Fibrosis in Unilateral Ureteral Obstruction Mice.
Wang B, Wang J, He W, Zhao Y, Zhang A, Liu Y, Hassounah F, Ma F, Klein JD, Wang XH, Wang H. Wang B, et al. Hum Gene Ther. 2020 Mar;31(5-6):367-375. doi: 10.1089/hum.2019.287. Epub 2020 Feb 21. Hum Gene Ther. 2020. PMID: 31950871 Free PMC article. - Extracellular vesicles: emerging roles, biomarkers and therapeutic strategies in fibrotic diseases.
Zhu J, Wang S, Yang D, Xu W, Qian H. Zhu J, et al. J Nanobiotechnology. 2023 May 24;21(1):164. doi: 10.1186/s12951-023-01921-3. J Nanobiotechnology. 2023. PMID: 37221595 Free PMC article. Review.
References
- Avram M.M., Mittman N. Malnutrition in uremia. Semin. Nephrol. 1994;14:238–244. - PubMed
- Griffiths R.D. Muscle mass, survival, and the elderly ICU patient. Nutrition. 1996;12:456–458. - PubMed
- Eddy A.A., Neilson E.G. Chronic kidney disease progression. J. Am. Soc. Nephrol. 2006;17:2964–2966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical