Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing - PubMed (original) (raw)
Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing
Sebastian Sjöqvist et al. J Extracell Vesicles. 2019.
Abstract
The oral mucosa exhibits unique regenerative properties, sometimes referred to as foetal-like wound healing. Researchers from our institute have used sheets of oral mucosa epithelial cells (OMECs) for regenerative medicine applications including cornea replacement and oesophageal epithelial regeneration for stricture prevention. Here, we have isolated exosomes from clinical-grade production of OMEC sheets from healthy human donors (n = 8), aiming to evaluate the clinical potential of the exosomes to stimulate epithelial regeneration and to improve understanding of the mode-of-action of the cells. Exosomes were isolated from conditioned (cExo) and non-conditioned (ncExo) media. Characterization was performed using Western blot for common exosomal-markers: CD9 and flotillin were positive while annexin V, EpCam and contaminating marker GRP94 were negative. Nanoparticle tracking analysis revealed a diameter of ~120 nm and transmission electron microscopy showed a corresponding size and spherical appearance. Human skin fibroblasts exposed to exosomes showed dose-dependent reduction of proliferation and a considerable increase of growth factor gene expression (HGF, VEGFA, FGF2 and CTGF). The results were similar for both groups, but with a trend towards a larger effect from cExo. To study adhesion, fluorescently labelled exosomes were topically applied to pig oesophageal wound-beds ex vivo and subsequently washed. Positive signal could be detected after as little as 1 min of adhesion, but increased adhesion time produced a stronger signal. Next, labelled exosomes were added to full-thickness skin wounds in rats and signal was detected up to 5 days after application. cExo significantly reduced the wound size at days 6 and 17. In conclusion, exosomes from OMEC sheets showed pro-regenerative effects on skin wound healing. This is the first time that the healing capacity of the oral mucosa is studied from an exosome perspective. These findings might lead to a combinational therapy of cell sheets and exosomes for future patients with early oesophageal cancer.
Keywords: Extracellular vesicles; clinical samples; exosomes; oral keratinocytes; regenerative medicine; therapy; wound healing.
Figures
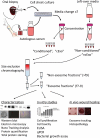
Figure 1.
Study overview. Serum and oral mucosal tissue were collected from healthy donors and epithelial cells were isolated and cultured as cell sheets. The autologous serum was used in the culture media and exosomes isolated from this media represent an important control in downstream analyses (“non-conditioned”, “ncExo”). Exosomes were also isolated from used media that was collected from the cell cultures (“conditioned”, “cExo”). For exosome isolation, the media was concentrated and subjected to size exclusion chromatography. The exosomes were then used for characterization, in vitro, and in vivo studies.
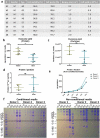
Figure 2.
Donor details and exosome isolation. (a) Details of healthy volunteers which have donated blood and oral mucosal tissue, and the outcome of cell isolation. (b) The yield, expressed as nanogram protein per millilitre media, was higher in non-conditioned media than in the conditioned media (n = 7). (c) The number of isolated particles per millilitre media (d) and the amount of protein per particle (e) was not significantly different between the groups (n = 3). (e) Protein quantification of different fractions from the size exclusion chromatography. Fractions 7–9 (“EV-fractions”) had considerably lower protein content, suggesting a successful separation from bulk non-EV-proteins. (f) Coomassie Brilliant Blue-staining of gel electrophoresis suggests that fractions 7–9 are almost completely void of contaminating proteins. The dotted rectangle indicates albumin (66.5 kDa), a common contaminant.
Figure 3.
Western blot analysis. Western blot was performed on cExo and ncExo samples, both from exosome-fractions (fractions 7–9) and from a non-exosome-fraction (fraction 14). Normal human keratinocyte lysate was used as control. (a) CD9 was positive in all exosome-samples and in the lysate, but negative in the non-exosome-fraction. (b) Flotillin was positive in all exosome-fractions and lysate, but with varying signal strength, and no signal was detected in the non-exosome-fraction. (c) Annexin V was negative in all fractions, but positive in the lysate. (d) EpCAM was negative in all samples (expected at 40 kDa, dotted box). (e) HSP70, heat shock protein 70, was negative in all samples except for cell lysate control. (f) GRP94, an endoplasmic reticulum protein that commonly contaminates vesicle isolates, was also negative in all samples except for cell lysate control. d3, d4 and d5 refer to donors 3, 4 and 5.
Figure 4.
Electron microscopy and nanoparticle tracking analysis. (a) Transmission electron microscopy revealed a rounded appearance from both groups of exosomes. (b) Nanoparticle tracking analysis showed a mean size of around 125 nm with no difference between the two groups (n = 4). Scale bar = 50 nm (left) and 500 nm (right).
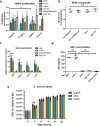
Figure 5.
In vitro bioactivity. (a) The exosomes were tested in a 72-h proliferation assay. At 0.2 µg/mL, cExo, but not ncExo significantly reduced the proliferation of cells, to the same extent as dexamethasone. At higher concentrations the difference between the groups was less (n = 12 for EV groups, n = 4–8 for control groups, * indicates significance difference to 0 µg/mL, † indicates significant difference to dexamethasone group). (b) Neither of the exosome groups nor dexamethasone showed any significant cytotoxicity (72 h with high density seeding, 2 µg/mL). (c) The gene expression of several wound-healing related genes was up-regulated after exposure to both types of exosomes. In general, cExo induced the expression more strongly than ncExo, but this was only significant for HGF. Dexamethasone suppressed the expression for all factors except for CTGF. (d) Stimulation with both groups of exosomes (2 µg/mL) led to an extensive increase of HGF-secretion from fibroblasts. (e) cExo had a small suppressive effect on S. aureus at the 3-h time point (2 µg/mL).
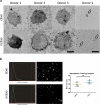
Figure 6.
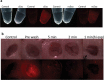
Fluorescence labelling and adhesion assay. (a) Exosomes from both groups could successfully be labelled with membrane-binding fluorescence dye. (b) Adhesion was tested in an ex vivo model using porcine oesophagus. The control tissue showed a weak auto fluorescence. Labelled exosomes were topically added and the tissue visualized under dissection microscope (“pre-wash”). The exosomes were allowed to adhere for 5, 2 or 1 min, after which the tissue was washed to remove unbound exosomes. The signal decreased with shorter adhesion time. After 1-min adhesion time, the signal could still be detected, but only with increased exposure time.
Figure 7.
Exosome tracking in vivo. (a) Labelled exosomes were topically applied to full-thickness wounds on rat dorsal sites. The exosomes were added just after the wound was made and on the following day. The signal could be detected up to day 4. On days 5 and 6 it was difficult to distinguish the signal due to auto fluorescence from re-grown hair and fibrin scab. (b) Using confocal microscopy on sections from the wounds at day 6, signal could be detected in 2/3 ncExo and 3/3 cExo samples. Scale bar = 100 µm.
Figure 8.
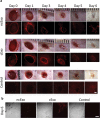
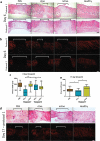
cExo stimulate wound healing. Two protocols were tried. In protocol 1, 7.6 µg exosomes were applied over the first 2 days. Protocol 2 involved applying 12.5 µg exosomes on day 0 only. (a) For protocol 1, H&E staining revealed a reduced wound size (brackets) and a largely restored histological architecture surrounding the wound area in the cExo group. In contrast, ncExo appears similar to the PBS group. Protocol 2 was considerably less effective with similar histopathology between the groups. (b) Polarized visualization of Picrosirius staining clearly shows the wound area (brackets) with less signal due to minimal collagen content. (c) Quantification of the wound sizes revealed a considerable decrease of wound size using cExo and protocol 1, significant from all other groups. (d) Protocol 1 on a 17-day time point revealed similar results, with a reduced wound size by cExo only, confirmed by blinded quantification (e). Scale bars = 200 µm.
Similar articles
- Oral keratinocyte-derived exosomes regulate proliferation of fibroblasts and epithelial cells.
Sjoqvist S, Kasai Y, Shimura D, Ishikawa T, Ali N, Iwata T, Kanai N. Sjoqvist S, et al. Biochem Biophys Res Commun. 2019 Jun 30;514(3):706-712. doi: 10.1016/j.bbrc.2019.04.202. Epub 2019 May 8. Biochem Biophys Res Commun. 2019. PMID: 31078263 - [Effects of human amniotic epithelial stem cells-derived exosomes on healing of wound with full-thickness skin defect in rats].
Zhao B, Wu GF, Zhang YJ, Zhang W, Yang FF, Xiao D, Zeng KX, Shi JH, Su LL, Hu DH. Zhao B, et al. Zhonghua Shao Shang Za Zhi. 2017 Jan 20;33(1):18-23. doi: 10.3760/cma.j.issn.1009-2587.2017.01.005. Zhonghua Shao Shang Za Zhi. 2017. PMID: 28103990 Chinese. - Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway.
Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z, Wang X, Luo L, Han F, Zhang J, Han S, Cai W, Su L, Tao K, Shi J, Hu D. Zhang W, et al. Exp Cell Res. 2018 Sep 15;370(2):333-342. doi: 10.1016/j.yexcr.2018.06.035. Epub 2018 Jun 28. Exp Cell Res. 2018. PMID: 29964051 - Mesenchymal Stem Cells-Derived Exosomes for Wound Regeneration.
Goodarzi P, Larijani B, Alavi-Moghadam S, Tayanloo-Beik A, Mohamadi-Jahani F, Ranjbaran N, Payab M, Falahzadeh K, Mousavi M, Arjmand B. Goodarzi P, et al. Adv Exp Med Biol. 2018;1119:119-131. doi: 10.1007/5584_2018_251. Adv Exp Med Biol. 2018. PMID: 30051320 Review. - Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair.
Bjørge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Bjørge IM, et al. Biomater Sci. 2017 Dec 19;6(1):60-78. doi: 10.1039/c7bm00479f. Biomater Sci. 2017. PMID: 29184934 Review.
Cited by
- SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling.
Wu M, Liu X, Li Z, Huang X, Guo H, Guo X, Yang X, Li B, Xuan K, Jin Y. Wu M, et al. Cell Prolif. 2021 Jul;54(7):e13074. doi: 10.1111/cpr.13074. Epub 2021 Jun 7. Cell Prolif. 2021. PMID: 34101281 Free PMC article. - New frontiers of oral sciences: Focus on the source and biomedical application of extracellular vesicles.
Yu W, Li S, Zhang G, Xu HHK, Zhang K, Bai Y. Yu W, et al. Front Bioeng Biotechnol. 2022 Oct 19;10:1023700. doi: 10.3389/fbioe.2022.1023700. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36338125 Free PMC article. Review. - Application of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets for Periodontal Regeneration.
Onizuka S, Iwata T. Onizuka S, et al. Int J Mol Sci. 2019 Jun 7;20(11):2796. doi: 10.3390/ijms20112796. Int J Mol Sci. 2019. PMID: 31181666 Free PMC article. Review. - Harnessing extracellular vesicle heterogeneity for diagnostic and therapeutic applications.
Carney RP, Mizenko RR, Bozkurt BT, Lowe N, Henson T, Arizzi A, Wang A, Tan C, George SC. Carney RP, et al. Nat Nanotechnol. 2025 Jan;20(1):14-25. doi: 10.1038/s41565-024-01774-3. Epub 2024 Oct 28. Nat Nanotechnol. 2025. PMID: 39468355 Free PMC article. Review. - Inhibition of Circulating Exosomal miRNA-20b-5p Accelerates Diabetic Wound Repair.
Chen K, Yu T, Wang X. Chen K, et al. Int J Nanomedicine. 2021 Jan 14;16:371-381. doi: 10.2147/IJN.S287875. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33469291 Free PMC article. Retracted.
References
- Mak K, Manji A, Gallant-Behm C, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–16. - PubMed
Grants and funding
This work was supported by the Swedish Society of Medicine, Erik och Edith Fernströms stiftelse för medicinsk forskning, Misao-Yanagihara-Grant for regenerative medicine research and JSPS KAKENHI [Grant Number JP18H02985].
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous