Resolving the Paradox of Hepatic Insulin Resistance - PubMed (original) (raw)
Review
Resolving the Paradox of Hepatic Insulin Resistance
Dominic Santoleri et al. Cell Mol Gastroenterol Hepatol. 2019.
Abstract
Insulin resistance is associated with numerous metabolic disorders, such as obesity and type II diabetes, that currently plague our society. Although insulin normally promotes anabolic metabolism in the liver by increasing glucose consumption and lipid synthesis, insulin-resistant individuals fail to inhibit hepatic glucose production and paradoxically have increased liver lipid synthesis, leading to hyperglycemia and hypertriglyceridemia. Here, we detail the intrahepatic and extrahepatic pathways mediating insulin's control of glucose and lipid metabolism. We propose that the interplay between both of these pathways controls insulin signaling and that mis-regulation between the 2 results in the paradoxic effects seen in the insulin-resistant liver instead of the commonly proposed deficiencies in particular branches of only the direct hepatic pathway.
Keywords: De Novo Lipogenesis; Hepatic Glucose Production; Hepatic Insulin Resistance; Insulin Signaling; Metabolism; PI3K/Akt Signaling Pathway.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
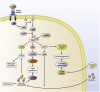
Figure 1
PI3K/Akt signaling in hepatocytes. Insulin binds to and activates the insulin receptor on the liver surface after a meal. After activation, the receptor recruits and activates IRS, which then activates PI3K. PI3K phosphorylates the signaling lipid molecule PIP2 into PIP3 in a process that is opposed by PTEN. PIP3 activates 3-phosphoinositide-dependent protein kinase 1 (PDK1), which phosphorylates Akt at Thr308. To fully activate Akt, mTORC2 also must phosphorylate it at Ser473. From Akt, different pathways for controlling glucose and lipid homeostasis branch out. Glycogen synthesis is induced through Akt inhibition of GSK3. In addition, Akt can promote glycogen synthesis in a manner independent of GSK3, such as activation of GYS2 by glucose-6-phosphate (G6P). Akt inhibition of TSC activates mTORC1, which in turn activates the lipogenic gene program through activation of SREBP1c and Gck, which phosphorylates glucose to G6P, which feeds into glycolysis and glycogen synthesis. In addition, G6P activates ChREBP, which activates lipogenesis along with SREBP1c. Akt inhibits FoxO1, resulting in an inhibition of gluconeogenesis by suppressing expressing of the proteins glucose-6-phosphatase (G6pc) and Pck1. Externally, FFAs can promote gluconeogenesis and contribute to insulin resistance by being taken up by the liver and converted to Acetyl-CoA, which activates pyruvate carboxylase.
Figure 2
Indirect insulin effects on the liver. Insulin can block gluconeogenesis and glycogenolysis in the liver through several indirect pathways. Its secretion from the pancreatic β cells inhibits glucagon secretion in neighboring α cells. In the adipose tissue, insulin inhibits lipolysis, which reduces the levels of circulating FFAs, which promote glucose production. Finally, insulin signaling in the hypothalamus and central nervous system acts on Agrp neurons to signal through the hepatic vagus branch to inhibit HGP.
References
- Zimmet P., Alberti K.G.M.M., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
- Brown M.S., Goldstein J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. - PubMed
- James O.F.W., Day C.P. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources