Genetics of Childhood Steroid Sensitive Nephrotic Syndrome: An Update - PubMed (original) (raw)
Review
Genetics of Childhood Steroid Sensitive Nephrotic Syndrome: An Update
Brandon M Lane et al. Front Pediatr. 2019.
Abstract
Advances in genome science in the last 20 years have led to the discovery of over 50 single gene causes and genetic risk loci for steroid resistant nephrotic syndrome (SRNS). Despite these advances, the genetic architecture of childhood steroid sensitive nephrotic syndrome (SSNS) remains poorly understood due in large part to the varying clinical course of SSNS over time. Recent exome and genome wide association studies from well-defined cohorts of children with SSNS identified variants in multiple MHC class II molecules such as HLA-DQA1 and HLA-DQB1 as risk factors for SSNS, thus stressing the central role of adaptive immunity in the pathogenesis of SSNS. However, evidence suggests that unknown second hit risk loci outside of the MHC locus and environmental factors also make significant contributions to disease. In this review, we examine what is currently known about the genetics of SSNS, the implications of recent findings on our understanding of pathogenesis of SSNS, and how we can utilize these results and findings from future studies to improve the management of children with nephrotic syndrome.
Keywords: HLA DQ/DR; MHC class II LOCUS; SSNS; nephrotic syndrome; podocyte.
Figures
Figure 1
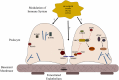
Genes associated with steroid sensitive nephrotic syndrome (SSNS). In the relatively rare cases of monogenic SSNS, the vast majority of genes that have been implicated in SSNS pathology locate to the glomerular filtration barrier and more specifically to the podocytes and slit diaphragm (Table 1). Among them are a key slit diaphragm protein Nephrin encoded by NPHS1 which is known to interact with MAGI2 and TNS2. These two proteins also interact as binding partners and along with CDK20 act to influence podocyte cytoskeletal activity through negative regulation of DLC1 activity (Red lines). DLC1 encodes s a RhoGTPase activating protein for RhoA and its activity can be modulated by the product of EMP2 through inhibition of the DLC1 binding partner Caveolin1 (CAV1). Intersectins 1 and 2 encoded by ITSN1 and ITSN2 act as GEFs for another small RhoGTPase, CDC42. The molecules encoded by KANK1 and KANK2 are also involved in RhoGTPase regulation and are known to affect actin polymerization. PLCE1 and PLCG2 encode proteins that modulate actin cytoskeleton and signaling at the slit diaphragm through calcium regulation. In addition to podocyte expression, PLCG2 is expressed in lymphocytes and it is known to affect immune response signaling. Evidence suggests that most cases of SSNS involve modulation of the immune system, though it is not clear exactly how this immune dysregulation affects the integrity of the glomerular filtration barrier. MHC Class II variants in the HLA-DR/DQ region are likely key components of this altered immune response. Other candidate loci include another MHC class II associated molecule BTNL2, as well as a regulator of T cell activity encoded by FOXP3. EXT1 is expressed in the glomerular basement membrane where it is involved in heparan sulfate biosynthesis.
Figure 2
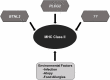
MHC Class II variants in SSNS. Accumulated genetic evidence has identified MHC Class II variants as a central component to SSNS pathogenesis. Additional risk variants such as those reported in BTNL2 and PLCG2, and other rare risk variants (race or none race specific) are likely to be involved as well. Environmental factors such as infection, atopy, and food allergies likely contribute to disease through increased activation of immune system.
Figure 3
Genetic and environmental contributions to NS. Evidence suggests that SSNS results from combination of immunological and environmental insults to podocyte in a genetic background that is susceptible to podocyte injury and that there is likely a negative correlation between therapy response in NS patients and the degree of genetic podocyte injury. In a simplified view of Nephrotic Syndrome, there is likely a threshold of podocyte injury at which glomerular filtration barrier integrity is lost (red line). In SRNS, the loss of GFB integrity results primarily from genetic insults to podocyte viability that is too profound to overcome with any beneficial cytoskeletal effects of immunosuppressants. Monogenic cases of SSNS likely have less genetic podocyte injury than SRNS, such that immunosuppressive therapy is largely successful at restoring GFB integrity but the overall podocyte viability is still damaged enough to allow environmental factors to influence remission status. More common and polygenic SSNS have a larger contribution of immunological insults to podocyte, which results in a more robust response to immunomodulatory therapy. Frequent relapsers likely have a higher level of baseline podocyte injury than infrequent relapsers.
Similar articles
- HLA-DQA1 and APOL1 as Risk Loci for Childhood-Onset Steroid-Sensitive and Steroid-Resistant Nephrotic Syndrome.
Adeyemo A, Esezobor C, Solarin A, Abeyagunawardena A, Kari JA, El Desoky S, Greenbaum LA, Kamel M, Kallash M, Silva C, Young A, Hunley TE, de Jesus-Gonzalez N, Srivastava T, Gbadegesin R. Adeyemo A, et al. Am J Kidney Dis. 2018 Mar;71(3):399-406. doi: 10.1053/j.ajkd.2017.10.013. Epub 2017 Dec 23. Am J Kidney Dis. 2018. PMID: 29277510 Free PMC article. - Genetics of childhood steroid-sensitive nephrotic syndrome.
Karp AM, Gbadegesin RA. Karp AM, et al. Pediatr Nephrol. 2017 Sep;32(9):1481-1488. doi: 10.1007/s00467-016-3456-8. Epub 2016 Jul 29. Pediatr Nephrol. 2017. PMID: 27470160 Free PMC article. Review. - HLA-DQA1 and PLCG2 Are Candidate Risk Loci for Childhood-Onset Steroid-Sensitive Nephrotic Syndrome.
Gbadegesin RA, Adeyemo A, Webb NJ, Greenbaum LA, Abeyagunawardena A, Thalgahagoda S, Kale A, Gipson D, Srivastava T, Lin JJ, Chand D, Hunley TE, Brophy PD, Bagga A, Sinha A, Rheault MN, Ghali J, Nicholls K, Abraham E, Janjua HS, Omoloja A, Barletta GM, Cai Y, Milford DD, O'Brien C, Awan A, Belostotsky V, Smoyer WE, Homstad A, Hall G, Wu G, Nagaraj S, Wigfall D, Foreman J, Winn MP; Mid-West Pediatric Nephrology Consortium. Gbadegesin RA, et al. J Am Soc Nephrol. 2015 Jul;26(7):1701-10. doi: 10.1681/ASN.2014030247. Epub 2014 Oct 27. J Am Soc Nephrol. 2015. PMID: 25349203 Free PMC article. - Association of HLA-DR/DQ alleles and haplotypes with nephrotic syndrome.
Ramanathan AS, Senguttuvan P, Chinniah R, Vijayan M, Thirunavukkarasu M, Raju K, Mani D, Ravi PM, Rajendran P, Krishnan JI, Karuppiah B. Ramanathan AS, et al. Nephrology (Carlton). 2016 Sep;21(9):745-52. doi: 10.1111/nep.12669. Nephrology (Carlton). 2016. PMID: 26566811 - An updated view of the pathogenesis of steroid-sensitive nephrotic syndrome.
Horinouchi T, Nozu K, Iijima K. Horinouchi T, et al. Pediatr Nephrol. 2022 Sep;37(9):1957-1965. doi: 10.1007/s00467-021-05401-4. Epub 2022 Jan 10. Pediatr Nephrol. 2022. PMID: 35006356 Free PMC article. Review.
Cited by
- Immunopathogenesis of idiopathic nephrotic syndrome.
Savas B, Fofana F, Le Gouvello S, Pawlak A, Sahali D, Ollero M. Savas B, et al. Cell Mol Immunol. 2022 Dec;19(12):1429-1431. doi: 10.1038/s41423-022-00908-8. Epub 2022 Aug 19. Cell Mol Immunol. 2022. PMID: 35986135 Free PMC article. No abstract available. - Mendelian steroid resistant nephrotic syndrome in childhood: is it as common as reported?
Arslan Z, Webb H, Ashton E, Foxler B, Tullus K, Waters A, Bockenhauer D. Arslan Z, et al. Pediatr Nephrol. 2023 Apr;38(4):1051-1056. doi: 10.1007/s00467-022-05569-3. Epub 2022 Jul 8. Pediatr Nephrol. 2023. PMID: 35802272 - Molecular Mechanisms of Proteinuria in Minimal Change Disease.
Purohit S, Piani F, Ordoñez FA, de Lucas-Collantes C, Bauer C, Cara-Fuentes G. Purohit S, et al. Front Med (Lausanne). 2021 Dec 23;8:761600. doi: 10.3389/fmed.2021.761600. eCollection 2021. Front Med (Lausanne). 2021. PMID: 35004732 Free PMC article. Review. - Estimation of childhood nephrotic syndrome incidence: data from the atlanta metropolitan statistical area and meta-analysis of worldwide cases.
Londeree J, McCracken CE, Greenbaum LA, Anderson EJ, Plantinga LC, Gillespie SE, Wang CS. Londeree J, et al. J Nephrol. 2022 Mar;35(2):575-583. doi: 10.1007/s40620-021-01108-9. Epub 2021 Jul 2. J Nephrol. 2022. PMID: 34213762 Free PMC article. - Prediction of steroid resistance and steroid dependence in nephrotic syndrome children.
Zaorska K, Zawierucha P, Świerczewska M, Ostalska-Nowicka D, Zachwieja J, Nowicki M. Zaorska K, et al. J Transl Med. 2021 Mar 30;19(1):130. doi: 10.1186/s12967-021-02790-w. J Transl Med. 2021. PMID: 33785019 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials