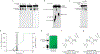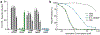Bacterial cGAS-like enzymes synthesize diverse nucleotide signals - PubMed (original) (raw)
Bacterial cGAS-like enzymes synthesize diverse nucleotide signals
Aaron T Whiteley et al. Nature. 2019 Mar.
Abstract
Cyclic dinucleotides (CDNs) have central roles in bacterial homeostasis and virulence by acting as nucleotide second messengers. Bacterial CDNs also elicit immune responses during infection when they are detected by pattern-recognition receptors in animal cells. Here we perform a systematic biochemical screen for bacterial signalling nucleotides and discover a large family of cGAS/DncV-like nucleotidyltransferases (CD-NTases) that use both purine and pyrimidine nucleotides to synthesize a diverse range of CDNs. A series of crystal structures establish CD-NTases as a structurally conserved family and reveal key contacts in the enzyme active-site lid that direct purine or pyrimidine selection. CD-NTase products are not restricted to CDNs and also include an unexpected class of cyclic trinucleotide compounds. Biochemical and cellular analyses of CD-NTase signalling nucleotides demonstrate that these cyclic di- and trinucleotides activate distinct host receptors and thus may modulate the interaction of both pathogens and commensal microbiota with their animal and plant hosts.
Conflict of interest statement
Competing interests: Harvard Medical School and the Dana-Farber Cancer Institute have patents pending for CD-NTase technologies on which the authors are inventors.
Figures
Extended Data Figure 1 |. Detailed characterization of CdnE, a cUMP–AMP synthase.
a and b, Titration of reaction buffer pH in steps of 0.2 pH units. Recombinant DncV or CdnE was incubated with α−32P radiolabeled ATP, CTP, GTP, and UTP at varying pH and the reactions were treated with alkaline phosphatase and visualized by PEI-cellulose TLC. CdnE activity is optimal at pH ~9.4, and this reaction condition was used in further experiments. Data are representative of 2 independent experiments. c, PEI-cellulose TLC of products after incubation of indicated enzyme, wild-type CdnE, or active site mutant CdnE with α−32P radiolabeled ATP, CTP, GTP, and UTP as in Fig. 1b. Mutations that ablate the CdnE Mg2+-coordinating, active-site residues eliminate all detectable activity. Data are representative of 3 independent experiments. d, Nuclease P1 sensitivity of CDN products. The endonuclease P1 specifically cleaves 3′–5′, canonical phosphodiester bonds. DncV and CdnE products are completely digested in the presence of P1 and alkaline phosphatase, whereas only one bond of 2′3′ cGAMP is susceptible to digestion, producing the linear G[2′–5′]pA product. Data are representative of 3 independent experiments. e, Workflow of nucleotide production for MS and NMR analysis. f, Anion exchange chromatography of a CdnE reaction with ATP and UTP, eluted with a gradient of Buffer B (2 M ammonium acetate) by FPLC. Individual fractions were concentrated prior to pooling for further analysis. g, Anion exchange chromatography (IEX) fractions from f were separated by silica TLC, visualized by UV shadowing, and compared to a radiolabeled reaction to confirm the appropriate A254 peak. Fractions were pooled and concentrated prior to MS and NMR analysis. h, k, and l, 3′3′ cyclic uridine monophosphate–adenosine monophosphate proton NMR spectra and associated zoomed in datasets. 1H NMR (400 MHz): δΗ 8.40 (s, 1H), 8.17 (s, 1H), 7.90 (d, J = 8.2 Hz, 1H), 6.15 (s, 1H), 5.75 (s, 1H), 5.55 (d, J = 8.2 Hz, 1H), 5.00–4.90 (m, 2H), 4.80 (d, J = 4.5 Hz, 1H) 4.70–4.61 (m, 1H), 4.55–4.38 (m, 4H), 4.17–4.02 (m, 2H) i and j, 3′3′ cyclic uridine monophosphate–adenosine monophosphate phosphate NMR spectra and associated zoomed in dataset. 31P{1H} NMR (162 MHz): δP −1.59 (s, 1P), −1.65 (s, 1P).
Extended Data Figure 2 |. DncV, cGAS, and CdnE reaction order.
a–c, Incubation of CD-NTase enzymes with nonhydrolyzable nucleotides traps reaction intermediates and identifies reaction order. Left, PEI-cellulose TLC analysis of reactions as in Fig. 1b where individual NTPs have been replaced with nonhydrolyzable nucleotide analogs. Exposed gamma phosphates are labeled in parenthesis to indicate these are removed by phosphatase treatment prior to analysis. Data are representative of 3 independent experiments. Right, previously determined reaction mechanisms (DncV and cGAS), and proposed reaction mechanism for CdnE.
Extended Data Figure 3 |. Detailed analysis of Rm-CdnE.
a, A thermophilic homolog of CdnE from Rhodothermus marinus (Rm-CdnE) also synthesizes cUMP–AMP. Recombinant proteins were incubated with α−32P radiolabeled NTPs as indicated at either 37 °C (CdnE) or 70 °C (Rm-CdnE) and the reactions were visualized by PEI-cellulose TLC as in Fig. 1b. Data are representative of 2 independent experiments. b, Active site of Rm-CdnE superimposed with structures of cGAS (6CTA) and DncV (4TY0). c, The analogous position to N166 was mutated in CdnE to a serine and that protein, CdnEN166S, was characterized in depth. Reactions analyzed as in Fig. 1b, demonstrate that CdnEN166S loses pyrimidine-specificity. Data are representative of 2 independent experiments. d, Structure-based sequence alignment of CD-NTases, annotated with secondary structure features of Rm-CdnE and human cGAS (6CTA). Mg2+ coordinating active site residues are highlighted in red, and the analogous residues to Rm-CdnE N166 are highlighted in orange.
Extended Data Figure 4 |. Detailed structural analysis of Em-CdnE.
a, Sequence alignment of CdnE homologs in Fig. 2c, annotated with Rm-CdnE secondary structure features. Mg2+ coordinating active site residues are highlighted in red, and the analogous residues to Rm-CdnE N166 are highlighted in orange. WP_050915017, Yersinia enterocolitica; WP_096075289, Pseudomonas aeruginosa; WP_104644370, Xanthomonas arboricola; WP_010848498, Xenorhabdus nematophila; WP_015040391, Bordetella parapertussis; WP_006482377, Burkholderia cepacia complex; WP_014072508, Rhodothermus marinus (Rm-CndE); WP_042646516, Legionella pneumophila; WP_062886322, Mycobacterium avium; WP_016200549, Elizabethkingia meningoseptica (Em-CdnE); WP_031901603, Staphylococcus aureus; WP_050492554, Enterococcus faecalis; WP_062695386, Bacteroides thetaiotaomicron. b, Biochemical deconvolution of Em-CdnE, which has a natural serine substitution at the N166 analogous site. Recombinant protein was incubated with NTPs as indicated and reactions were visualized as in Fig. 1b. Data are representative of 3 independent experiments. c, Reactions of Em-CdnE incubated with α−32P radiolabeled NTPs and nonhydrolyzable nucleotide analogs as indicated, and visualized as in Fig. 1b. Data are representative of 3 independent experiments. d, Anion exchange chromatography of an Em-CdnE reaction with ATP and GTP, eluted with a gradient of Buffer B (2 M ammonium acetate) by FPLC. Individual fractions were concentrated prior to pooling for further analysis. e, Anion exchange chromatography (IEX) fractions from d were separated by silica TLC, visualized by UV shadowing, and compared to a radiolabeled reaction to confirm the appropriate peak. Fractions were pooled and concentrated prior to MS analysis. MS confirmed synthesis of products with masses corresponding to c-di-AMP, cGAMP, and c-di-GMP. f, Crystal structure of Em-CdnE in complex with GTP and nonhydrolyzable ATP capturing the “1st state” structure prior to NTP hydrolysis. Mg2+ ions are omitted for clarity. g, Zoom-in cut-away of the active site of f, confirming position of a serine at the analogous site to Rm-CdnE N166. Mg2+ ions are shown in green. Nucleotide and metal 2Fo−Fc electron density is contoured at 1 σ. h, Zoom-in cut-away of the active site of Em-CdnE–pppApA structure, capturing the “2nd state” after the first reaction has occurred to form a linear intermediate, but prior to CDN formation. Mg2+ ions are shown in green. Nucleotide and metal 2Fo−Fc electron density is contoured at 1 σ. i, Biochemical deconvolution of mutant Em-CdnE reverted to the ancestral asparagine at the N166 analogous site. This mutant loses preference for producing cyclic dipurine molecules and instead produces more pyrimidine-containing CDN products. Reactions were visualized as in Fig. 1b. Data are representative of 2 independent experiments.
Extended Data Figure 5 |. cUMP–AMP-recognition helps define innate immune receptor specificity.
a and e, Quantification of nucleotide interactions with the host receptors STING or RECON, measured with radiolabeled nucleotide bound to a concentration gradient of host protein, separated in a native PAGE gel shift (0, 4, 20, 100 µM protein). Data are quantification of gels in b and f and representative of n=2 independent experiments. b- and f, Native PAGE gel shift analysis of STING or RECON complex formation with indicated radiolabeled CDNs. Proteins are titrated at 0 (–), 4, 20, and 100 µM. For quantification see a and e. STING readily binds all cyclic dipurine species but does not form a high-affinity complex with cUMP–AMP. RECON readily binds all 3′3′ CDN species that contain at least one adenine base, including cUMP–AMP. Data are representative of 2 independent experiments. c, In-cell STING reporter assay. Induction of an IFN-β reporter in HEK293T cells transfected with a concentration gradient of plasmid overexpressing enzymes as indicated. DncV and CdnE were expressed with N-terminal MBP tags and IFN-β reporter induction was compared as fold over empty vector, shown as (−). Data are mean ± SEM for n=3 technical replicates, and are representative of 2 independent experiments. d, Western blot of MBP-tagged DncV and CdnE expressed from plasmids analyzed in c to validate in vivo expression. Data are representative of 2 independent experiments. Gel source data are available as Supplementary Figure 1. g, Gel shift analysis as in f, with protein titration to measure the relative affinity of the RECON–cUMP–AMP interaction. Protein concentrations listed below. Data are representative of 2 independent experiments.
Extended Data Figure 6 |. A biochemical screen of CD-NTases from diverse bacterial genera.
a-, Chart of the number of bacterial genomes (N = a total 16,717) that harbor CD-NTases from clusters in Fig. 4a. See also Supplementary Table 2. b-, Taxa of genome sequenced bacteria isolated with unique CD-NTase genes, bold face type indicate phyla, Proteobacteria and Firmicutes are further divided by order and visualized by shades of color. c-f-, Type CD-NTases interrogated for product synthesis. Purified proteins were incubated with α−32P radiolabeled NTPs under different reaction conditions (indicated pH and divalent cation) and reaction products were visualized by either PEI-cellulose or Silica TLC as in Fig. 1b and Fig. 4c. g, CD-NTase expression level and purity. Coomassie stained SDS-PAGE analysis of purified CD-NTase enzymes used in each reaction. Data are representative of 2 independent experiments.
Extended Data Figure 7 |. Detailed biochemical analysis of Lp-CdnE02.
a-, Biochemical deconvolution of Lp-CdnE02 (CD-NTase057) as in Fig. 1c, demonstrates specific synthesis of cyclic dipyrimidine products. Data are representative of 3 independent experiments. b, Nuclease sensitivity of the Lp-CdnE02 product, as described in Extended Data Fig. 1d. Data are representative of 3 independent experiments. c, Incubation of Lp-CdnE02 with nonhydrolyzable nucleotides, as described in Extended Data Fig. 2. Nonhydrolyzable UTP completely blocks the reaction, indicating the first step requires attack of the α-P from UTP. However, the product formed when nonhydrolyzable CTP is present cannot be distinguished from c-di-UMP in this assay, and it is unclear if the reaction proceeds through a pppCpU reaction intermediate. Data are representative of 3 independent experiments. d, Anion exchange chromatography of a Lp-CdnE02 reaction with UTP and CTP, eluted with a gradient of Buffer B (2 M ammonium acetate) by FPLC. Individual fractions were concentrated prior to pooling for further analysis. e, Anion exchange chromatography (IEX) fractions from d were separated by silica TLC, visualized by UV shadowing, and compared to a radiolabeled reaction to confirm the appropriate peak. Fractions were pooled and concentrated prior to MS analysis. f, MS confirmed synthesis of c-di-UMP as the major product of Lp-CdnE02. g, MS confirmed synthesis of cCMP–UMP as a minor product of Lp-CdnE02.
Extended Data Figure 8 |. CD-NTases are encoded in conserved, poorly understood operons on mobile genetic elements.
a-, Operon structure for CD-NTases selected for in-depth characterization showing the conserved protein domains in CD-NTase adjacent genes (see Fig. 4a–c). Conserved operons were first identified by Burroughs et al. and operons are vertically organized by similarity to one another. Where found, linked genes demonstrating CD-NTases are encoded on mobile genetic elements are indicated. b, CD-NTases and their adjacently encoded “effector” proteins were coexpressed in E. coli and bacterial colony formation was quantified by spot dilution analysis. CD-NTases were inducibly expressed from a chloramphenicol resistant (CmR) vector and effectors were inducibly expressed from a carbenicillin resistant (CarbR) vector. Bacteria harboring cognate CD-NTase / effector plasmids or control plasmids were plated on medium containing both inducers and incubated for 24 h at 37 °C. Data were not determined (N.D.) for CD-NTase036 because the effector was toxic to E. coli under non-inducing conditions. Data are the mean ± SEM of 3 independent experiments. c, Spot dilution analysis of bacteria harboring the cognate CD-NTase-effector pair indicated, as quantified in b. The CD-NTase036-effector pair was not analyzed in this assay. Colony morphology indicates a potential interaction for some combinations, however, it is unclear how specific or significant this may be. Data are representative of 3 independent experiments.
Extended Data Figure 9 |. Detailed biochemical analysis of Ec-CdnD02.
a-, Titration of reaction buffer pH in steps of 0.2 pH units. Recombinant Ec-CdnD02 was incubated with α−32P radiolabeled NTPs at varying pH and the reactions were analyzed and visualized by PEI-cellulose or silica TLC as in Fig. 1b and Fig. 4c. Silica TLC identified two products, denoted the major (blue triangle) and minor (red triangle) product. Quantification of TLC spots is shown below. Data are representative of 2 independent experiments. b-, Biochemical deconvolution of Ec-CdnD02, recombinant protein was incubated with NTPs as indicated and analyzed by TLC as in a. Data are representative of 3 independent experiments. c-, Nuclease digestion of the Ec-CdnD02 product. Conventional nuclease digestion includes addition of a phosphatase, in this experiment reactions were first treated with Antarctic phosphatase to remove remaining NTPs then heat inactivated. Next, reactions were either untreated, treated with nuclease P1 (specific for 3′–5′ phosphodiester bonds) only, or treated with nuclease P1 and phosphatase to remove exposed phosphate groups. 3′3′ cGAMP (DncV) and Ec-CdnD02 product are digested into AMP and GMP constituents, which are phosphatase sensitive. cAMP (CyaA) is insensitive to P1 digestion and cyclic monophosphates are phosphatase resistant. These data demonstrate that the Ec-CdnD02 product does not contain a cyclic monophosphate. Data are representative of 3 independent experiments. d, Incubation of Ec-CdnD02 with nonhydrolyzable nucleotides, as described in Extended Data Fig. 2. Nonhydrolyzable ATP completely blocks the reaction, indicating the first step requires attack of the α-P from ATP. However, when nonhydrolyzable GTP is present the possible intermediates [pp(c)pGpA, pp(c)pGpApA, or pppApA] cannot be distinguished in this assay and it is unclear how the reaction proceeds. Silica TLC is not suited for analyzing nonhydrolyzable nucleotides because they do not migrate beyond the origin. Data are representative of 3 independent experiments. e, Anion exchange chromatography of a Ec-CdnD02 reaction with ATP and GTP, eluted with a gradient of Buffer B (2 M ammonium acetate) by FPLC. Individual fractions were concentrated prior to pooling for further analysis. f and g, 3′3′3′ tricyclic adenosine monophosphate–adenosine monophosphate–guanosine monophosphate (cAAG) NMR spectra and associated zoomed in dataset. 31P{1H} NMR (162 MHz): δP −0.65 (s, 1P), −0.70 (s, 1P), −0.75 (s, 1P). h-j, 3′3′3′ tricyclic adenosine monophosphate–adenosine monophosphate–guanosine monophosphate (cAAG) proton NMR spectra and associated zoomed in datasets. 1H NMR (400 MHz): δΗ 8.43 (s, 1H), 8.39 (s, 1H), 8.19 (s, 1H), 8.12 (s, 1H), 8.01 (s, 1H), 6.15 (d, J = 7.0 Hz, 1H), 6.12 (d, J = 7.0 Hz, 1H), 5.92 (d, J = 7.5 Hz, 1H), 5.00–4.78 (m, 6H), 4.69–4.58 (m, 3H), 4.3–4.2 (m, 6H).
Extended Data Figure 10 |. Structural analysis of cAAG inhibition of RECON.
a, cAAG interactions with STING or RECON, radiolabeled nucleotide incubated with a concentration gradient of each protein, separated in a native PAGE gel shift (0, 4, 20, 100 µM protein). Data are representative of 2 independent experiments. b, Co-crystal structure of the RECON–cAAG complex shown as cartoon (left) and surface (right). c, Overlay and orientation of RECON ligands cAAG, c-di-AMP (5UXF), co-substrate NAD (3LN3) demonstrate three individual binding pockets. d, Schematic representation of residues from RECON that interact with cAAG. Green dotted lines indicate hydrogen bonding, grey dotted lines indicate hydrophobic interactions. e, Zoom-in cutaways of individual RECON binding pockets as in d. f, Mammalian innate-immune sensors recognize CD-NTase products with overlapping specificities. 2′3′ cGAMP and c-di-GMP are detected by STING; 3′3′ cGAMP and c-di-AMP are detected by both STING and RECON; and cUMP–AMP and cAAG are detected by RECON.
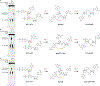
Figure 1 |. Bacteria synthesize cyclic UMP–AMP.
a, An E. coli genomic island homologous to the Vibrio seventh pandemic island-I (VSP-I) encodes a 3′3′ cGAMP synthase (dncV) and phospholipase receptor (capV). The ECOR31 island encodes a second _capV_-like gene (BHF03_01995 encoding WP_001593459, renamed capE) adjacent to a gene of unknown function (BHF03_01990 encoding WP_001593458, renamed cdnE). b, PEI-cellulose TLC of enzyme reactions. Purified CdnE was incubated with α−32P radiolabeled ATP, CTP, GTP, and UTP. Reactions were terminated by treating with alkaline phosphatase to remove exposed triphosphates. Standards are 2′3′ cGAMP (cGAS), 3′3′ cGAMP (DncV), c-di-AMP (DisA), and c-di-GMP (WspR). Data are representative of 3 independent experiments. c, Biochemical deconvolution of the CdnE reactions as in (b) after incubating with α−32P labeled and unlabeled NTPs. Data are representative of 3 independent experiments. d, The CdnE product, confirmed by mass spectrometry (MS) and NMR (see Extended Data Fig. 1e–l). e, Activation of CapV and CapE by CDNs, tested with no nucleotide added (−) or at 0.1, 1, and 10-fold molar ratios of nucleotide to phospholipase. Enzyme activity is reported in Phospholipase A1 units mL−1. Data are mean ± standard error of the mean (SEM) for n=3 technical replicates and are representative of 3 independent experiments.
Figure 2 |. Conserved active site residues dictate CD-NTase specificity.
a, Crystal structure of Rm-CdnE in complex with nonhydrolyzable ATP and UTP analogs and zoom-in inset of key N166–uridine contacts controlling pyrimidine specificity. Green dotted lines indicate hydrogen bonding and 2Fo−Fc electron density map is contoured at 1 σ. b, Phylogram of CdnE sequence homologs and their N166 analogous residue determined by sequence alignment (Extended Data Fig. 4a). Red “S’s” highlight cGAS / DncV-like serine residues. c, CdnE homolog and mutant reactions analyzed by TLC as in Fig. 1b. “N” vs red “S” indicate asparagine or cGAS / DncV-like serine at the N166 analogous position in the tested allele. Side-chains are numbered according to Rm-CdnE sequence. Data are representative of 3 independent experiments. For detailed deconvolution and purine vs pyrimidine migration pattern analysis see Extended Data Fig. 3 and 4. d, Structure-based comparisons define that Rm-CdnE and Em-CdnE are cGAS / DncV-like nucleotidyltransferase (CD-NTases) with a similar architecture to DncV (4TY0), cGAS (6CTA), and OAS1 (4RWO). CD-NTases are more distantly related to Pol-β-like NTases: Pol-μ (4YD1), Pol-β (4KLQ), CCA-adding enzyme (4X4T), and Poly(A) Polymerase gamma (PAP, 4LT6). The NTase core domain for each enzyme is clustered according to Z-score and colored in magenta/blue.
Figure 3 |. Immune detection of a pyrimidine containing CDN.
a, In-cell STING reporter assay. Induction of an Interferon-β (IFN-β) reporter in HEK293T cells transfected with a concentration gradient of plasmid overexpressing enzymes as indicated. cGAS synthesizes 2′3′ cGAMP, DncV synthesizes 3′3′ cGAMP, DisA synthesizes c-di-AMP, WspR synthesizes c-di-GMP, and CdnE synthesizes cUMP–AMP. Data are fold induction over vector only, shown as (−), are mean ± SEM for n=3 technical replicates, and are representative of 3 independent experiments. b, Nucleotide inhibition of RECON enzymatic activity, as measured by oxidation of NADPH co-substrate. X-axis is a log scale and data are representative of 3 independent experiments.
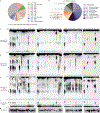
Figure 4 |. CD-NTases synthesize diverse nucleotide products and form a family of enzymes abundant in many bacterial phyla.
a, Bioinformatic identification and alignment of ~5,600 predicted CD-NTases found in nearly every bacterial phylum, shown as an unrooted tree. Sequence-related enzymes that are ~10% identical are grouped by lettered clade and similarly colored, enzymes ~25% identical are grouped by cluster in a similar color shade. Circles represent CD-NTase001–066 that were selected as type CD-NTases for a biochemical screen (See Extended Data Fig 6 for additional details). Blue circles denote CD-NTases selected for in-depth characterization and are labeled with CD-NTase numbers from the biochemical screen (see Fig. 4b, CdnE is “56” and DncV is “D”). For additional information, see Supplementary Discussion, Supplementary Table 2, and source data for this figure provided as Supplementary Data. b and c, PEI-cellulose and silica TLC analysis of 16 CD-NTases selected for in-depth characterization. Activity was analyzed with α−32P radiolabeled NTPs as in Fig. 1b. Wild-type (WT) and catalytically inactive mutant (mt) DncV reactions are included as controls. Screened CD-NTases were numbered CD-NTase001–066. CD-NTase056 is CdnE, CD-NTase057 was renamed Lp-CdnE02, and CD-NTase038 was renamed Ec-CdnD02. These appear as “56”, “57”, and “38” in Fig. 4a, respectively. Data are representative of 3 independent experiments. d, Identification of CD-NTase products by combining TLC and MS data. CD-NTases that synthesize a major product that could not be matched with a predicted CDNs are denoted as “unknown.”
Figure 5 |. Bacterial synthesis and host recognition of a cyclic trinucleotide.
a, Silica TLC analysis of the Ec-CdnD02 (CD-NTase038) product and control reactions as in Fig. 1b. The major product of Ec-CdnD02 is indicated with a triangle and incorporates ~2× greater α−32P from ATP than α−32P from GTP. Data are representative of 3 independent experiments. b, The major product of Ec-CdnD02, cyclic AMP–AMP–GMP (cAAG), confirmed by MS and NMR, see Extended Data Fig. 9 for additional characterization. c, cAAG inhibition of RECON enzymatic activity, as measured by oxidation of NADPH co-substrate. X-axis is a log scale and data are representative of 3 independent experiments. d, Co-crystal structure of the host receptor RECON in complex with cAAG, and inset highlighting the cAAG 2Fo−Fc electron density contoured at 1.3 σ. Green dotted lines indicate hydrogen bonding, some RECON–cAAG contacts omitted for clarity, see Extended Data Fig. 10.
Similar articles
- Crystal structure and functional implications of cyclic di-pyrimidine-synthesizing cGAS/DncV-like nucleotidyltransferases.
Yang CS, Ko TP, Chen CJ, Hou MH, Wang YC, Chen Y. Yang CS, et al. Nat Commun. 2023 Aug 21;14(1):5078. doi: 10.1038/s41467-023-40787-9. Nat Commun. 2023. PMID: 37604815 Free PMC article. - Molecular basis of CD-NTase nucleotide selection in CBASS anti-phage defense.
Govande AA, Duncan-Lowey B, Eaglesham JB, Whiteley AT, Kranzusch PJ. Govande AA, et al. Cell Rep. 2021 Jun 1;35(9):109206. doi: 10.1016/j.celrep.2021.109206. Cell Rep. 2021. PMID: 34077735 - cGAS and CD-NTase enzymes: structure, mechanism, and evolution.
Kranzusch PJ. Kranzusch PJ. Curr Opin Struct Biol. 2019 Dec;59:178-187. doi: 10.1016/j.sbi.2019.08.003. Epub 2019 Oct 6. Curr Opin Struct Biol. 2019. PMID: 31593902 Free PMC article. Review. - Crystal structure and functional implication of a bacterial cyclic AMP-AMP-GMP synthetase.
Ko TP, Wang YC, Tsai CL, Yang CS, Hou MH, Chen Y. Ko TP, et al. Nucleic Acids Res. 2021 May 7;49(8):4725-4737. doi: 10.1093/nar/gkab165. Nucleic Acids Res. 2021. PMID: 33836064 Free PMC article. - CBASS phage defense and evolution of antiviral nucleotide signaling.
Duncan-Lowey B, Kranzusch PJ. Duncan-Lowey B, et al. Curr Opin Immunol. 2022 Feb;74:156-163. doi: 10.1016/j.coi.2022.01.002. Epub 2022 Feb 2. Curr Opin Immunol. 2022. PMID: 35123147 Review.
Cited by
- (p)ppGpp and c-di-AMP Homeostasis Is Controlled by CbpB in Listeria monocytogenes.
Peterson BN, Young MKM, Luo S, Wang J, Whiteley AT, Woodward JJ, Tong L, Wang JD, Portnoy DA. Peterson BN, et al. mBio. 2020 Aug 25;11(4):e01625-20. doi: 10.1128/mBio.01625-20. mBio. 2020. PMID: 32843560 Free PMC article. - Bacterial cGAS senses a viral RNA to initiate immunity.
Banh DV, Roberts CG, Morales-Amador A, Berryhill BA, Chaudhry W, Levin BR, Brady SF, Marraffini LA. Banh DV, et al. Nature. 2023 Nov;623(7989):1001-1008. doi: 10.1038/s41586-023-06743-9. Epub 2023 Nov 15. Nature. 2023. PMID: 37968393 Free PMC article. - STING Targeting in Lung Diseases.
de Moura Rodrigues D, Lacerda-Queiroz N, Couillin I, Riteau N. de Moura Rodrigues D, et al. Cells. 2022 Nov 3;11(21):3483. doi: 10.3390/cells11213483. Cells. 2022. PMID: 36359882 Free PMC article. Review. - Ubiquitin-like conjugation by bacterial cGAS enhances anti-phage defence.
Jenson JM, Li T, Du F, Ea CK, Chen ZJ. Jenson JM, et al. Nature. 2023 Apr;616(7956):326-331. doi: 10.1038/s41586-023-05862-7. Epub 2023 Feb 27. Nature. 2023. PMID: 36848932 Free PMC article. - Second messengers and divergent HD-GYP phosphodiesterases regulate 3',3'-cGAMP signaling.
Wright TA, Jiang L, Park JJ, Anderson WA, Chen G, Hallberg ZF, Nan B, Hammond MC. Wright TA, et al. Mol Microbiol. 2020 Jan;113(1):222-236. doi: 10.1111/mmi.14412. Epub 2019 Nov 17. Mol Microbiol. 2020. PMID: 31665539 Free PMC article.
References
- Wu J & Chen ZJ Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol 32, 461–488 (2014). - PubMed
- Ross P et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281 (1987). - PubMed
Methods References
- Studier FW Protein production by auto-induction in high density shaking cultures. Protein Expression and Purification 41, 207–234 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD021527/OD/NIH HHS/United States
- T32 CA207021/CA/NCI NIH HHS/United States
- S10 OD021832/OD/NIH HHS/United States
- P30 GM124165/GM/NIGMS NIH HHS/United States
- R01 AI026289/AI/NIAID NIH HHS/United States
- R37 AI018045/AI/NIAID NIH HHS/United States
- R01 AI018045/AI/NIAID NIH HHS/United States
- S10 RR029205/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous