Safety and Immunogenicity of a 2-Dose Heterologous Vaccine Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Nairobi, Kenya - PubMed (original) (raw)
Clinical Trial
Safety and Immunogenicity of a 2-Dose Heterologous Vaccine Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Nairobi, Kenya
Gaudensia Mutua et al. J Infect Dis. 2019.
Abstract
Background: During the 2014 West African Ebola outbreak, Ebola vaccine development was accelerated. The phase 1 VAC52150EBL1003 study was performed to investigate 2-dose heterologous vaccination with Ad26.ZEBOV and MVA-BN-Filo in an African population located in a high-altitude setting in Nairobi, Kenya.
Methods: Healthy adult volunteers were randomized to receive one of four 2-dose vaccination schedules. The first vaccination was administered at baseline (Ad26.ZEBOV or MVA-BN-Filo), followed by the second vaccination with the alternate vaccine after either 28 or 56 days. Each schedule had a placebo comparator group. The primary objective was to assess the safety and tolerability of these regimens.
Results: Seventy-two volunteers were randomized into 4 groups of 18 (15 received vaccine, and 3 received placebo). The most frequent solicited systemic adverse event was headache (frequency, 50%, 61%, and 42% per dose for MVA-BN-Filo, Ad26.ZEBOV, and placebo, respectively). The most frequent solicited local AE was injection site pain (frequency, 78%, 63%, and 33% per dose for MVA-BN-Filo, Ad26.ZEBOV, and placebo, respectively). No differences in adverse events were observed among the different vaccine regimens. High levels of binding and neutralizing anti-Ebola virus glycoprotein antibodies were induced by all regimens and sustained to day 360 after the first dose.
Conclusions: Two-dose heterologous vaccination with Ad26.ZEBOV and MVA-BN-Filo was well tolerated and highly immunogenic against Ebola virus glycoprotein.
Clinical trials registration: NCT02376426.
Keywords: Ad26.ZEBOV; Ebola vaccine; MVA-BN-Filo; heterologous 2-dose; safety and immunogenicity.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
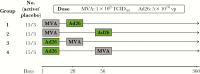
Figure 1.
VAC52150EBL1003 study design. Ad26, Ad26.ZEBOV; MVA, MVA-BN-Filo; TCID50, 50% tissue culture infectious doses; vp, virus particle.
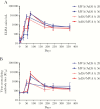
Figure 2.
Anti–Ebola virus glycoprotein immunoglobulin G binding antibody responses (detected by enzyme-linked immunosorbent assay [ELISA]) binding antibody responses (A) and virus neutralizing antibody (VNA) responses (B) following dose 1 vaccination with Ad26.ZEBOV (Ad26) or MVA-BN-Filo (MVA) and heterologous dose 2 vaccination with MVA or Ad26 on day 29 or day 57, 21 days after dose 2. Data are geometric mean concentration (GMC), for ELISA, and geometric mean 50% inhibitory concentration (IC50), for VNA analysis. Error bars represent 95% confidence intervals. NA, not applicable.
Figure 3.
Durability of anti–Ebola virus glycoprotein immunoglobulin G binding (A) and neutralizing (B) antibody responses following dose 1 vaccination with Ad26.ZEBOV (Ad26) or MVA-BN-Filo (MVA) and heterologous dose 2 vaccination with MVA or Ad26 on day 29 or day 57. Data are geometric mean values; error bars represent 95% confidence intervals. ELISA, enzyme-linked immunosorbent assay; IC50, 50% inhibitory concentration.
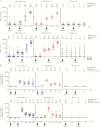
Figure 4.
Median CD8+ T-cell responses (A) and CD4+ T-cell responses (B) following first dose vaccination with Ad26.ZEBOV (Ad26) or MVA-BN-Filo (MVA) and heterologous second dose vaccination with MVA or Ad26 on day 29 or day 57.
Similar articles
- Safety and Immunogenicity of a 2-Dose Heterologous Vaccination Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Uganda and Tanzania.
Anywaine Z, Whitworth H, Kaleebu P, Praygod G, Shukarev G, Manno D, Kapiga S, Grosskurth H, Kalluvya S, Bockstal V, Anumendem D, Luhn K, Robinson C, Douoguih M, Watson-Jones D. Anywaine Z, et al. J Infect Dis. 2019 Jun 5;220(1):46-56. doi: 10.1093/infdis/jiz070. J Infect Dis. 2019. PMID: 30796818 Free PMC article. Clinical Trial. - Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial.
Ishola D, Manno D, Afolabi MO, Keshinro B, Bockstal V, Rogers B, Owusu-Kyei K, Serry-Bangura A, Swaray I, Lowe B, Kowuor D, Baiden F, Mooney T, Smout E, Köhn B, Otieno GT, Jusu M, Foster J, Samai M, Deen GF, Larson H, Lees S, Goldstein N, Gallagher KE, Gaddah A, Heerwegh D, Callendret B, Luhn K, Robinson C, Leyssen M, Greenwood B, Douoguih M, Leigh B, Watson-Jones D; EBL3001 study group. Ishola D, et al. Lancet Infect Dis. 2022 Jan;22(1):97-109. doi: 10.1016/S1473-3099(21)00125-0. Epub 2021 Sep 13. Lancet Infect Dis. 2022. PMID: 34529963 Free PMC article. Clinical Trial. - Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial.
Pollard AJ, Launay O, Lelievre JD, Lacabaratz C, Grande S, Goldstein N, Robinson C, Gaddah A, Bockstal V, Wiedemann A, Leyssen M, Luhn K, Richert L, Bétard C, Gibani MM, Clutterbuck EA, Snape MD, Levy Y, Douoguih M, Thiebaut R; EBOVAC2 EBL2001 study group. Pollard AJ, et al. Lancet Infect Dis. 2021 Apr;21(4):493-506. doi: 10.1016/S1473-3099(20)30476-X. Epub 2020 Nov 17. Lancet Infect Dis. 2021. PMID: 33217361 Clinical Trial. - The Brighton Collaboration standardized template for collection of key information for risk/benefit assessment of a Modified Vaccinia Ankara (MVA) vaccine platform.
Volkmann A, Williamson AL, Weidenthaler H, Meyer TPH, Robertson JS, Excler JL, Condit RC, Evans E, Smith ER, Kim D, Chen RT; Brighton Collaboration Viral Vector Vaccines Safety Working Group V3SWG. Volkmann A, et al. Vaccine. 2021 May 21;39(22):3067-3080. doi: 10.1016/j.vaccine.2020.08.050. Epub 2020 Oct 17. Vaccine. 2021. PMID: 33077299 Free PMC article. Review. - Ebola virus disease: current vaccine solutions.
Tomori O, Kolawole MO. Tomori O, et al. Curr Opin Immunol. 2021 Aug;71:27-33. doi: 10.1016/j.coi.2021.03.008. Epub 2021 Apr 17. Curr Opin Immunol. 2021. PMID: 33873076 Review.
Cited by
- Safety and Immunogenicity of Heterologous and Homologous 2-Dose Regimens of Adenovirus Serotype 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized, Controlled Phase 1 Study.
Goldstein N, Bockstal V, Bart S, Luhn K, Robinson C, Gaddah A, Callendret B, Douoguih M. Goldstein N, et al. J Infect Dis. 2022 Sep 4;226(4):595-607. doi: 10.1093/infdis/jiaa586. J Infect Dis. 2022. PMID: 32939546 Free PMC article. Clinical Trial. - Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases.
Wang S, Liang B, Wang W, Li L, Feng N, Zhao Y, Wang T, Yan F, Yang S, Xia X. Wang S, et al. Signal Transduct Target Ther. 2023 Apr 7;8(1):149. doi: 10.1038/s41392-023-01408-5. Signal Transduct Target Ther. 2023. PMID: 37029123 Free PMC article. Review. - Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate.
Roozendaal R, Hendriks J, van Effelterre T, Spiessens B, Dekking L, Solforosi L, Czapska-Casey D, Bockstal V, Stoop J, Splinter D, Janssen S, Baelen BV, Verbruggen N, Serroyen J, Dekeyster E, Volkmann A, Wollmann Y, Carrion R Jr, Giavedoni LD, Robinson C, Leyssen M, Douoguih M, Luhn K, Pau MG, Sadoff J, Vandebosch A, Schuitemaker H, Zahn R, Callendret B. Roozendaal R, et al. NPJ Vaccines. 2020 Dec 17;5(1):112. doi: 10.1038/s41541-020-00261-9. NPJ Vaccines. 2020. PMID: 33335092 Free PMC article. - Implementation of accelerated research: strategies for implementation as applied in a phase 1 Ad26.ZEBOV, MVA-BN-Filo two-dose Ebola vaccine clinical trial in Uganda.
Kitonsa J, Ggayi AB, Anywaine Z, Kisaakye E, Nsangi L, Basajja V, Nyantaro M, Watson-Jones D, Shukarev G, Ilsbroux I, Robinson C, Kaleebu P. Kitonsa J, et al. Glob Health Action. 2020 Dec 31;13(1):1829829. doi: 10.1080/16549716.2020.1829829. Glob Health Action. 2020. PMID: 33073737 Free PMC article. Clinical Trial. - Antibody-Dependent Natural Killer Cell Activation After Ebola Vaccination.
Wagstaffe HR, Clutterbuck EA, Bockstal V, Stoop JN, Luhn K, Douoguih M, Shukarev G, Snape MD, Pollard AJ, Riley EM, Goodier MR. Wagstaffe HR, et al. J Infect Dis. 2021 Apr 8;223(7):1171-1182. doi: 10.1093/infdis/jiz657. J Infect Dis. 2021. PMID: 31821493 Free PMC article.
References
- Centers for Disease Control and Prevention (CDC). 2014 Ebola outbreak in West Africa—case counts 2016. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed April 2018.
- Milligan ID, Gibani MM, Sewell R, et al. . Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 2016; 315:1610–23. - PubMed
- Winslow RL, Milligan ID, Voysey M, et al. . Immune responses to novel adenovirus type 26 and modified vaccinia virus ankara-vectored Ebola vaccines at 1 year. JAMA 2017; 317:1075–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical