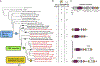Eukaryotic Acquisition of a Bacterial Operon - PubMed (original) (raw)
. 2019 Mar 7;176(6):1356-1366.e10.
doi: 10.1016/j.cell.2019.01.034. Epub 2019 Feb 21.
Drew T Doering 2, Dana A Opulente 1, Xing-Xing Shen 3, Xiaofan Zhou 4, Jeremy DeVirgilio 5, Amanda B Hulfachor 6, Marizeth Groenewald 7, Mcsean A Mcgee 8, Steven D Karlen 9, Cletus P Kurtzman 5, Antonis Rokas 3, Chris Todd Hittinger 10
Affiliations
- PMID: 30799038
- PMCID: PMC7295392
- DOI: 10.1016/j.cell.2019.01.034
Eukaryotic Acquisition of a Bacterial Operon
Jacek Kominek et al. Cell. 2019.
Abstract
Operons are a hallmark of bacterial genomes, where they allow concerted expression of functionally related genes as single polycistronic transcripts. They are rare in eukaryotes, where each gene usually drives expression of its own independent messenger RNAs. Here, we report the horizontal operon transfer of a siderophore biosynthesis pathway from relatives of Escherichia coli into a group of budding yeast taxa. We further show that the co-linearly arranged secondary metabolism genes are expressed, exhibit eukaryotic transcriptional features, and enable the sequestration and uptake of iron. After transfer, several genetic changes occurred during subsequent evolution, including the gain of new transcription start sites that were sometimes within protein-coding sequences, acquisition of polyadenylation sites, structural rearrangements, and integration of eukaryotic genes into the cluster. We conclude that the genes were likely acquired as a unit, modified for eukaryotic gene expression, and maintained by selection to adapt to the highly competitive, iron-limited environment.
Keywords: Saccharomycotina; Starmerella; Wickerhamiella; budding yeasts; central dogma of biology; enterobactin; horizontal gene transfer; operon; siderophore biosynthesis.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests:
The authors declare no competing interests.
Figures
Figure 1.. Distribution of the iron uptake and storage systems among fungi.
Plus (green) and minus (orange) signs indicate the presence and absence of iron uptake and storage systems in specific taxonomic groups. The numbers in parentheses (green) indicate the number of species in a taxonomic group that possess a specific system, if it is not ubiquitous in that group. The blue box indicates the budding yeasts. RIA - Reductive Iron Assimilation. IRGF - Iron-Responsive GATA Factor. Asterisks (*) mark paraphyletic groups. Note that only Wickerhamiella/Starmerella (W/S) clade fungi contain the bacterial or catecholate-class siderophore biosynthesis pathway, whereas many other dikaryon fungi contain hydroxamate-class siderophore biosynthesis pathways. See also Figure S1 and Table S1.
Figure 2.. Yeast siderophore biosynthesis originated from an Enterobacteriales lineage.
(A) ML phylogeny from the super-alignment of entABCDE genes from 207 Gammaproteobacteria and 12 yeasts, rooted at the midpoint. Bootstrap support values are shown for relevant branches within the Enterobacteriales (red). Other Gammaproteobacteria are blue.(B) Detailed view of the yeast clade from the main phylogeny, with bootstrap support values. (C) Alternative scenarios for the horizontal operon transfer. (D) P-values of the AU tests of different evolutionary hypotheses; EO - Enterobacteriales origin; non-EO - non-Enterobacteriales origin; 12-mono - 12 yeast sequences are monophyletic, 11-mono - 11 yeast sequences monophyletic and one unconstrained (12 alternatives tested, lowest p-value shown, full details in Table S2); 5G - topology of the yeast clade constrained to the one inferred from the super-alignment of entABCDE genes. Shimodaira-Hasegawa (SH) tests had less statistical power but produced fully concordant results. See also Table S1 and Table S2.
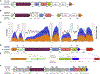
Figure 3.. Evolution of the siderophore biosynthesis genes in yeasts.
(A) ML phylogeny reconstructed from the concatenated alignment of 661 conserved, single copy genes (834,750 sites), with branch support values below 100 shown. Strains in bold denote genomes sequenced in this study, while strains in red denote genomes containing the siderophore biosynthesis genes. Black diamonds indicate secondary losses in yeast lineages, accompanied by losses of the siderophore importer ARN genes, which are often found in close proximity. (1) Horizontal operon transfer from an Enterobacteriales lineage. (2) Rearrangement and integration of genes encoding ferric reductase (FRE) and an uncharacterized transmembrane protein (TM). (3) Disruption by integration of the SNZ-SNO gene pair and translocation. (B) Species-specific data on presence/absence of the siderophore biosynthesis genes and experimental evidence for the presence of enterobactin in the yeast cultures as determined by an O-CAS assay (not specific to enterobactin) and direct chemical detection by HPLC-MS/MS (enterobactin produced by E. coli was used as the standard; nt - not tested). Note that culture conditions between assays were not identical, and siderophore expression is often condition-dependent (Machuca and Milagres, 2003). (C) Genetic structure of the siderophore biosynthesis operon in E. coli and yeasts, drawn to scale. Individual colors represent homologous genes, and gray marks bacterial genes not found in yeasts. Black circles represent contig termini within 25kb. See also Figure S4 and Table S4.
Figure 4.. Transcriptomics of the siderophore biosynthesis genes in W. versatilis.
(A, B, D) Diagram of siderophore biosynthesis genes as present in the genomes of St. bombicola (A), St. apicola (B), and W. versatilis (D), drawn to scale. Counts above the diagram indicate read-pairs that map to both predicted protein-coding sequences (low, non-zero read counts are likely DNA contamination). Counts below indicate the size of intergenic regions between adjacent protein-coding sequences, in base pairs. (C) The orange area indicates per-base coverage by RNA-Seq reads (read coverage). The blue area indicates per-base cumulative coverage by RNA-Seq reads and inserts between read-pairs (span coverage). The black line indicates the ratio of the read coverage over the span coverage, which is expected to remain ~50% in the middle of gene transcripts and rise towards 100% at transcript termini. Thus, transcript boundaries are visualized as a coverage trough between two spikes that approach 100% ratios. Ratios below 100% at the putative 5’ or 3’ ends of annotated transcripts, coupled with non-zero coverage of their intergenic regions, suggest overlapping or potentially bicistronic transcripts. The expected 3’ coverage bias can be observed for individual transcripts in the raw coverage data. (E) Results of 5′ and 3′ RACE experiments, depicting the positions of all detected m7G caps (green vertical lines) and poly(A) tails (red vertical lines) in the entB-entD (left) and entA-entH (right) gene pairs in W. versatilis. The outer and inner gene-specific primers are marked by diagonal black lines and were used along with outer and inner primers specific to the 5’ or 3’ RACE adapters provided in the kit (see Materials and Methods), which were adjacent to either the 5’ m7G cap or the 3’ poly(A) tail, respectively. Dotted lines indicate sequences amplified only during the outer nested RACE PCR step, while solid lines indicate the portions of the transcripts that were amplified during the inner nested RACE PCR step, and which were subsequently cloned for sequencing (F) Diagram of siderophore biosynthesis genes as present in the E. coli genome drawn to scale. Counts above the diagram indicate read-pairs crossmapping between genes (based on data from Seo et al., 2014, complete coverage maps shown in Figure S6). Counts below indicate the size of intergenic regions between adjacent protein-coding sequences, in base pairs (negative numbers indicate overlap). The f prefix (fA-fG) indicates the fepA-fepG genes. See also Figure S2, Figure S3 and Table S3.
Comment in
- Some Like it HOT: Horizontal Operon Transfer.
Lindsey ARI, Newton ILG. Lindsey ARI, et al. Cell. 2019 Mar 7;176(6):1243-1245. doi: 10.1016/j.cell.2019.02.007. Cell. 2019. PMID: 30849369
Similar articles
- Functional and Evolutionary Integration of a Fungal Gene With a Bacterial Operon.
Sun L, David KT, Wolters JF, Karlen SD, Gonçalves C, Opulente DA, LaBella AL, Groenewald M, Zhou X, Shen XX, Rokas A, Hittinger CT. Sun L, et al. Mol Biol Evol. 2024 Apr 2;41(4):msae045. doi: 10.1093/molbev/msae045. Mol Biol Evol. 2024. PMID: 38415839 Free PMC article. - Multilayered horizontal operon transfers from bacteria reconstruct a thiamine salvage pathway in yeasts.
Gonçalves C, Gonçalves P. Gonçalves C, et al. Proc Natl Acad Sci U S A. 2019 Oct 29;116(44):22219-22228. doi: 10.1073/pnas.1909844116. Epub 2019 Oct 14. Proc Natl Acad Sci U S A. 2019. PMID: 31611373 Free PMC article. - Evolution of mosaic operons by horizontal gene transfer and gene displacement in situ.
Omelchenko MV, Makarova KS, Wolf YI, Rogozin IB, Koonin EV. Omelchenko MV, et al. Genome Biol. 2003;4(9):R55. doi: 10.1186/gb-2003-4-9-r55. Epub 2003 Aug 29. Genome Biol. 2003. PMID: 12952534 Free PMC article. - Selfish operons: the evolutionary impact of gene clustering in prokaryotes and eukaryotes.
Lawrence J. Lawrence J. Curr Opin Genet Dev. 1999 Dec;9(6):642-8. doi: 10.1016/s0959-437x(99)00025-8. Curr Opin Genet Dev. 1999. PMID: 10607610 Review. - Evidence against the selfish operon theory.
Pál C, Hurst LD. Pál C, et al. Trends Genet. 2004 Jun;20(6):232-4. doi: 10.1016/j.tig.2004.04.001. Trends Genet. 2004. PMID: 15145575 Review.
Cited by
- SARS-CoV-2 envelope protein alters calcium signaling via SERCA interactions.
Berta B, Tordai H, Lukács GL, Papp B, Enyedi Á, Padányi R, Hegedűs T. Berta B, et al. Sci Rep. 2024 Sep 11;14(1):21200. doi: 10.1038/s41598-024-71144-5. Sci Rep. 2024. PMID: 39261533 Free PMC article. - Distribution and comparative genomic analysis of antimicrobial gene clusters found in Pantoea.
Kirk A, Stavrinides J. Kirk A, et al. Front Microbiol. 2024 Aug 14;15:1416674. doi: 10.3389/fmicb.2024.1416674. eCollection 2024. Front Microbiol. 2024. PMID: 39206372 Free PMC article. - Rapid functional activation of horizontally transferred eukaryotic intron-containing genes in the bacterial recipient.
Yuan W, Yu J, Li Z. Yuan W, et al. Nucleic Acids Res. 2024 Aug 12;52(14):8344-8355. doi: 10.1093/nar/gkae628. Nucleic Acids Res. 2024. PMID: 39011898 Free PMC article. - Comparative genomics of the closely related fungal genera Cryptococcus and Kwoniella reveals karyotype dynamics and suggests evolutionary mechanisms of pathogenesis.
Coelho MA, David-Palma M, Shea T, Bowers K, McGinley-Smith S, Mohammad AW, Gnirke A, Yurkov AM, Nowrousian M, Sun S, Cuomo CA, Heitman J. Coelho MA, et al. PLoS Biol. 2024 Jun 6;22(6):e3002682. doi: 10.1371/journal.pbio.3002682. eCollection 2024 Jun. PLoS Biol. 2024. PMID: 38843310 Free PMC article. - Extensive remodeling of sugar metabolism through gene loss and horizontal gene transfer in a eukaryotic lineage.
Pontes A, Paraíso F, Silva M, Lagoas C, Aires A, Brito PH, Rosa CA, Lachance MA, Sampaio JP, Gonçalves C, Gonçalves P. Pontes A, et al. BMC Biol. 2024 May 30;22(1):128. doi: 10.1186/s12915-024-01929-7. BMC Biol. 2024. PMID: 38816863 Free PMC article.
References
- Adeolu M, Alnajar S, Naushad S, Gupta RS (2016). Genome-based phylogeny and taxonomy of the “Enterobacteriales”: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol 66, 5575–5599. - PubMed
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. J. Mol. Biol 215, 403–410. - PubMed
- Andrews SC, Robinson AK, Rodríguez-Quiñones F (2003). Bacterial iron homeostasis. FEMS Microbiol. Rev 27, 215–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials