Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: A potential target for cancer treatment - PubMed (original) (raw)
Review
. 2019 May;176(9):1190-1205.
doi: 10.1111/bph.14632. Epub 2019 Apr 3.
Affiliations
- PMID: 30801705
- PMCID: PMC6468257
- DOI: 10.1111/bph.14632
Review
Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: A potential target for cancer treatment
Chaochu Cui et al. Br J Pharmacol. 2019 May.
Abstract
Due to its Ca2+ buffering capacity, the mitochondrion is one of the most important intracellular organelles in regulating Ca2+ dynamic oscillation. Mitochondrial calcium uniporter (MCU) is the primary mediator of Ca2+ influx into mitochondria, manipulating cell energy metabolism, ROS production, and programmed cell death, all of which are critical for carcinogenesis. The understanding of the uniporter complex was significantly boosted by recent groundbreaking discoveries that identified the uniporter pore-forming subunit MCU and its regulatory molecules, including MCU-dominant negative β subunit (MCUb), essential MCU regulator (EMRE), MCU regulator 1 (MCUR1), mitochondrial calcium uptake (MICU) 1, MICU2, and MICU3. These provide the means and molecular platform to investigate MCU complex (uniplex)-mediated impaired Ca2+ signalling in physiology and pathology. This review aims to summarize the progress of the understanding regulatory mechanisms of uniplex, roles of uniplex-mediated Ca2+ signalling in cancer, and potential pharmacological inhibitors of MCU.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
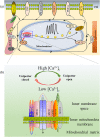
Figure 1
Uniplex components and uniplex‐mediated mitochondrial Ca2+ uptake in the global Ca2+ dynamic. (a) The dynamics of the intracellular Ca2+ oscillation are finely regulated by the mitochondria, SR/ER, lysosome, and channels located on the PM. When Ca2+ is transported from the extracellular space or released from intracellular Ca2+ stores, mitochondria will move close to the corresponding channel to take up the elevated [Ca2+]c, thus propagate or restrict Ca2+ signals to specific cellular domains. Ca2+ influx into mitochondria is mainly mediated by the uniplex. (b) Uniplex is composed of the pore‐forming subunit MCU, MCUb, EMRE, MCUR1, MICU1, MICU2, and MICU3. All these molecules localize at the inner mitochondrial membrane. MICU family protein or EMRE molecule contains only single TMD. Both MCU and MCUb contain two TMDs, while both N‐ and C‐terminals face the mitochondrial matrix. With two TMDs, N‐ and C‐terminals of MCUR1 face the mitochondria inner membrane space
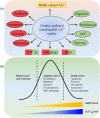
Figure 2
Functions of uniplex‐mediated mitochondrial Ca2+ uptake. (a) Effect of uniplex‐mediated Ca2+ signalling. It buffers the elevated [Ca2+]c, thus transduces or restricts calcium oscillation coded signalling. [Ca2+]m binding to specific proteins directly induces biochemical events, including cell migration, proliferation, survival, ATP production, autophagy, ROS, apoptosis, necrosis, necroptosis, and senescence. Autophagy, [Ca2+]m, and ROS are multifunctional events or factors. (b) Dual roles of [Ca2+]m and mROS. [Ca2+]m and mROS are required for normal cell functions and signalling. Under moderate stress, the increased Ca2+ and ROS could enhance the corresponding signalling pathway for adapting to the elevated requirement of ATP, proliferation, and autophagy. Once this promoted adaptive stress is out of control, it may lead to tumourigenesis and cell invasion. However, once [Ca2+]m and mROS accumulation exceed the threshold, death‐related events, such as apoptosis and strong autophagy, will be induced. Manipulation of these processes can regulate the fate
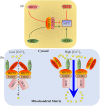
Figure 3
Regulatory mechanisms of uniplex. (a) MCUR1 and MCUb directly bind to MCU, which exerts a positive and dominant negative effect on MCU, respectively. MICU2 is a gatekeeper and MICU3 is an enhancer of MCU, both of which require MICU1‐EMRE heterodimer as a bridge. (b) EMRE directly binds to MICU1 and MCU. At low [Ca2+]c levels, both MICU2 and MICU3 bind to MICU1, and MICU2 inhibits MCU activation. When [Ca2+]c increases and exceeds the Ca2+ threshold set by MICU2, the MICU1–MICU2 dimer will dissociate from the uniplex, and MICU2 will lose its inhibitory function; MICU3 enhances MCU activity to transfer Ca2+ from the cytosol into the mitochondrial matrix
Similar articles
- The mitochondrial calcium uniporter: Balancing tumourigenic and anti-tumourigenic responses.
Colussi DM, Stathopulos PB. Colussi DM, et al. J Physiol. 2024 Jul;602(14):3315-3339. doi: 10.1113/JP285515. Epub 2024 Jun 10. J Physiol. 2024. PMID: 38857425 Review. - Molecular nature and physiological role of the mitochondrial calcium uniporter channel.
Alevriadou BR, Patel A, Noble M, Ghosh S, Gohil VM, Stathopulos PB, Madesh M. Alevriadou BR, et al. Am J Physiol Cell Physiol. 2021 Apr 1;320(4):C465-C482. doi: 10.1152/ajpcell.00502.2020. Epub 2020 Dec 9. Am J Physiol Cell Physiol. 2021. PMID: 33296287 Free PMC article. Review. - EMRE is an essential component of the mitochondrial calcium uniporter complex.
Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. Sancak Y, et al. Science. 2013 Dec 13;342(6164):1379-82. doi: 10.1126/science.1242993. Epub 2013 Nov 14. Science. 2013. PMID: 24231807 Free PMC article. - Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex.
Tsai MF, Phillips CB, Ranaghan M, Tsai CW, Wu Y, Willliams C, Miller C. Tsai MF, et al. Elife. 2016 Apr 21;5:e15545. doi: 10.7554/eLife.15545. Elife. 2016. PMID: 27099988 Free PMC article. - Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex.
Fan M, Zhang J, Tsai CW, Orlando BJ, Rodriguez M, Xu Y, Liao M, Tsai MF, Feng L. Fan M, et al. Nature. 2020 Jun;582(7810):129-133. doi: 10.1038/s41586-020-2309-6. Epub 2020 May 20. Nature. 2020. PMID: 32494073 Free PMC article.
Cited by
- Mitochondrial calcium uptake regulates tumour progression in embryonal rhabdomyosarcoma.
Chiu HY, Loh AHP, Taneja R. Chiu HY, et al. Cell Death Dis. 2022 Apr 30;13(4):419. doi: 10.1038/s41419-022-04835-4. Cell Death Dis. 2022. PMID: 35490194 Free PMC article. - Key Role of MCUR1 in Malignant Progression of Breast Cancer.
Gao P, Peng T, Lin S, Zhi W, Cao C, Wu P, Xi L, Wu P, Yang Q, Ding W. Gao P, et al. Onco Targets Ther. 2021 Jul 13;14:4163-4175. doi: 10.2147/OTT.S306854. eCollection 2021. Onco Targets Ther. 2021. PMID: 34285508 Free PMC article. - Targeting mitochondrial ion channels for cancer therapy.
Szabo I, Zoratti M, Biasutto L. Szabo I, et al. Redox Biol. 2021 Jun;42:101846. doi: 10.1016/j.redox.2020.101846. Epub 2020 Dec 24. Redox Biol. 2021. PMID: 33419703 Free PMC article. Review. - Application of calcium overload-based ion interference therapy in tumor treatment: strategies, outcomes, and prospects.
Li S, Fan R, Wang Y, He K, Xu J, Li H. Li S, et al. Front Pharmacol. 2024 Feb 15;15:1352377. doi: 10.3389/fphar.2024.1352377. eCollection 2024. Front Pharmacol. 2024. PMID: 38425645 Free PMC article. Review. - Store-Operated Ca2+ Entry in Tumor Progression: From Molecular Mechanisms to Clinical Implications.
Chen YF, Lin PC, Yeh YM, Chen LH, Shen MR. Chen YF, et al. Cancers (Basel). 2019 Jun 27;11(7):899. doi: 10.3390/cancers11070899. Cancers (Basel). 2019. PMID: 31252656 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous