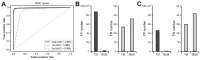Dual deep neural network-based classifiers to detect experimental seizures - PubMed (original) (raw)
Dual deep neural network-based classifiers to detect experimental seizures
Hyun-Jong Jang et al. Korean J Physiol Pharmacol. 2019 Mar.
Abstract
Manually reviewing electroencephalograms (EEGs) is labor-intensive and demands automated seizure detection systems. To construct an efficient and robust event detector for experimental seizures from continuous EEG monitoring, we combined spectral analysis and deep neural networks. A deep neural network was trained to discriminate periodograms of 5-sec EEG segments from annotated convulsive seizures and the pre- and post-EEG segments. To use the entire EEG for training, a second network was trained with non-seizure EEGs that were misclassified as seizures by the first network. By sequentially applying the dual deep neural networks and simple pre- and post-processing, our autodetector identified all seizure events in 4,272 h of test EEG traces, with only 6 false positive events, corresponding to 100% sensitivity and 98% positive predictive value. Moreover, with pre-processing to reduce the computational burden, scanning and classifying 8,977 h of training and test EEG datasets took only 2.28 h with a personal computer. These results demonstrate that combining a basic feature extractor with dual deep neural networks and rule-based pre- and post-processing can detect convulsive seizures with great accuracy and low computational burden, highlighting the feasibility of our automated seizure detection algorithm.
Keywords: Deep learning; Epilepsy; Mice; Seizures; Spectral analysis.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Figures
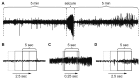
Fig. 1. Data set preparation for deep learning.
(A) Non-seizure data were obtained from 5 min of electroencephalogram (EEG) before and after each seizure. (B) Half overlapping 5-sec sliding windows were used to collect non-seizure data. (C) Five-sec segments per 0.25 sec were collected for seizure data to multiply the scarce seizure data. (D) While autodetecting seizure events, the entire EEG was scanned with half overlapping 5-sec sliding windows.
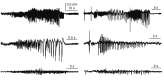
Fig. 2. Examples of convulsive seizures.
Seizure activities have different shapes, amplitudes, and durations. Our goal was to determine whether deep learning is adequate to detect different seizure activities.
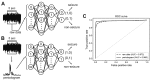
Fig. 3. Classification process for 5-sec electroencephalogram (EEG) segments that were either raw or subjected to spectral analysis.
(A) A classifier was built to distinguish total 5,000 raw EEG inputs from 5-sec EEG segments. (B) A classifier was built to distinguish total 100 periodogram results between 0 to 99 Hz. (C) Receiver operating characteristics (ROC) curve of classifiers that learned to distinguish seizure events from either raw data or periodogram results. Area under the curve (AUC) was calculated for each classifier.
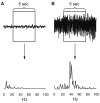
Fig. 4. Examples of the periodogram results for 0 to 99 Hz range.
(A) Non-seizure segment. (B) Seizure segment.
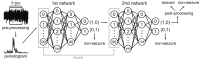
Fig. 5. Classification process for each 5-sec segment.
Each 5-sec segment underwent pre-processing to remove large noise and reduce unnecessary computation. The first 100 periodogram values in a 5-sec segment were fed to first deep neural network containing 100 input, 200 and 50 hidden, and 2 output nodes. If it was classified as a seizure, the second network, which contained 100 input, 500 and 125 hidden, and 2 output nodes, classified the same data again. If the second network also classified it as seizure, post-processing finally determined it as seizure if it passed a simple rule-based test. Non-seizure results at any point classified the data as non-seizure and started a new turn with next 5-sec segment.
Fig. 6. Improvement of classification performance by sequential dual deep neural networks.
(A) Receiver operating characteristics (ROC) curve and the area under the curve (AUC) when the 5-sec segments of whole training electroencephalogram datasets were classified with the first network only, the second network only, or sequential dual networks. (B) False positive (FP) and false negative (FN) segment numbers were compared after the application of the first network only or sequential dual networks when cut-off threshold for seizures was 0.5 and 0.5 for each network, respectively. (C) FP and FN segment numbers were compared after the application of the first network only or sequential dual networks when cut-off threshold for seizures was 0.99 and 0.90 for each network, respectively.
Fig. 7. Effects of network size and window size on seizure event detection results.
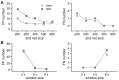
(A) Effects of network size. Left panel: false positive (FP) event numbers for training and test datasets for 200, 300, 400, 500, and 600 nodes in the first hidden layer of the second deep neural network. The number of nodes in the second hidden layer was one-fourth that of the first hidden layer. Right panel: false negative (FN) event numbers for training and test datasets for 200, 300, 400, 500, and 600 nodes in the first hidden layer of the second deep neural network. (B) Effects of window size. Left panel: FP event numbers in the training and test datasets for 2-, 5-, and 8-sec windows. Right panel: FN event numbers in the training and test datasets for 2-, 5-, and 8-sec windows.
Similar articles
- Six-Center Assessment of CNN-Transformer with Belief Matching Loss for Patient-Independent Seizure Detection in EEG.
Peh WY, Thangavel P, Yao Y, Thomas J, Tan YL, Dauwels J. Peh WY, et al. Int J Neural Syst. 2023 Mar;33(3):2350012. doi: 10.1142/S0129065723500120. Epub 2023 Feb 22. Int J Neural Syst. 2023. PMID: 36809996 - Automatic Seizure Detection Based on S-Transform and Deep Convolutional Neural Network.
Liu G, Zhou W, Geng M. Liu G, et al. Int J Neural Syst. 2020 Apr;30(4):1950024. doi: 10.1142/S0129065719500242. Epub 2019 Sep 30. Int J Neural Syst. 2020. PMID: 31564174 - A multistage knowledge-based system for EEG seizure detection in newborn infants.
Aarabi A, Grebe R, Wallois F. Aarabi A, et al. Clin Neurophysiol. 2007 Dec;118(12):2781-97. doi: 10.1016/j.clinph.2007.08.012. Epub 2007 Oct 1. Clin Neurophysiol. 2007. PMID: 17905654 - Deep learning for electroencephalogram (EEG) classification tasks: a review.
Craik A, He Y, Contreras-Vidal JL. Craik A, et al. J Neural Eng. 2019 Jun;16(3):031001. doi: 10.1088/1741-2552/ab0ab5. Epub 2019 Feb 26. J Neural Eng. 2019. PMID: 30808014 Review. - Automated seizure prediction.
Acharya UR, Hagiwara Y, Adeli H. Acharya UR, et al. Epilepsy Behav. 2018 Nov;88:251-261. doi: 10.1016/j.yebeh.2018.09.030. Epub 2018 Oct 11. Epilepsy Behav. 2018. PMID: 30317059 Review.
Cited by
- Barefoot walking improves cognitive ability in adolescents.
Kim T, Seo DY, Bae JH, Han J. Kim T, et al. Korean J Physiol Pharmacol. 2024 Jul 1;28(4):295-302. doi: 10.4196/kjpp.2024.28.4.295. Korean J Physiol Pharmacol. 2024. PMID: 38926837 Free PMC article. - Semi-automated seizure detection using interpretable machine learning models.
Antonoudiou P, Basu T, Maguire J. Antonoudiou P, et al. Res Sq [Preprint]. 2024 May 30:rs.3.rs-4361048. doi: 10.21203/rs.3.rs-4361048/v1. Res Sq. 2024. PMID: 38854086 Free PMC article. Preprint. - Deep Brain Stimulation Can Differentiate Subregions of the Human Subthalamic Nucleus Area by EEG Biomarkers.
Sand D, Arkadir D, Abu Snineh M, Marmor O, Israel Z, Bergman H, Hassin-Baer S, Israeli-Korn S, Peremen Z, Geva AB, Eitan R. Sand D, et al. Front Syst Neurosci. 2021 Oct 20;15:747681. doi: 10.3389/fnsys.2021.747681. eCollection 2021. Front Syst Neurosci. 2021. PMID: 34744647 Free PMC article. - A Recent Investigation on Detection and Classification of Epileptic Seizure Techniques Using EEG Signal.
Saminu S, Xu G, Shuai Z, Abd El Kader I, Jabire AH, Ahmed YK, Karaye IA, Ahmad IS. Saminu S, et al. Brain Sci. 2021 May 20;11(5):668. doi: 10.3390/brainsci11050668. Brain Sci. 2021. PMID: 34065473 Free PMC article. Review. - Artificial Intelligence and Computational Approaches for Epilepsy.
An S, Kang C, Lee HW. An S, et al. J Epilepsy Res. 2020 Jun 30;10(1):8-17. doi: 10.14581/jer.20003. eCollection 2020 Jun. J Epilepsy Res. 2020. PMID: 32983950 Free PMC article. Review.
References
- World Health Organization. Epilepsy Fact Sheet. Geneva: World Health Organization; 2018.
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. - PubMed
- Pitkänen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl 1):16–25. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources