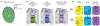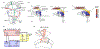Coordination of neural patterning in the Drosophila visual system - PubMed (original) (raw)
Review
Coordination of neural patterning in the Drosophila visual system
Maximilien Courgeon et al. Curr Opin Neurobiol. 2019 Jun.
Abstract
Precise formation of neuronal circuits requires the coordinated development of the different components of the circuit. Here, we review examples of coordination at multiples scales of development in one of the best-studied systems for neural patterning and circuit assembly, the Drosophila visual system, from coordination of gene expression in photoreceptors to the coordinated patterning of the different neuropiles of the optic lobe.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
Nothing declared.
Figures
Figure 1:
Fate specification in the retina: (a) Organization of the retinal mosaic. Yellow (y) and pale (p) ommatidia are randomly distributed in the retina, whereas DRA photoreceptors are localized in the last row of ommatidia at the dorsal margin of the retina (b) The three ommatidium subtypes and their Rhodopsin (Rh) expression. (c) Gene regulatory network controlling ommatidia subtype specification.
Figure 2:
Building the retinotopic map in the lamina: (a) Retinotopic organization of the visual system. Colors represent three different retinotopic points along the A-P axis. Examples of neurons from the different neuropiles that are retinotopically organized (L2 in the lamina, Mi1 in the medulla and T4 and T5 in the lobula complex). (b) Sequence of lamina differentiation during larval development: the entry of photoreceptor axons into the lamina lead to the division of Lamina Precursors Cells (LPCs) and their assembly into lamina columns. This is followed by the entry of wrapping glia cell processes that will direct the differentiation of LPCs into differentiated lamina monopolar cells. As the eye disc grows more photoreceptor axons (in red) and glial cells enter the lamina leading to more columns being assembled posteriorly following a front of differentiation. Note that L5 neurons at the bottom of the lamina follow a different sequence than the other lamina neurons and do not depend on signaling from wrapping glia. (c) Schematic of the signaling activities required for the formation and differentiation of lamina neurons. (d) Schematic of the adult lamina. Note that each lamina cartridge is composed of one copy of each of the 5 lamina monopolar cells and 6 outer photoreceptors (R1-6).
Figure 3:
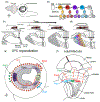
Medulla development: (a) Representation of the larval brain showing the OPC lying on the lateral surface of the brain, below the eye disc. (b) In the OPC neuroepithelium cells are converted into neuroblasts that express a temporal sequence of transcription factors that generates neuronal diversity. During each window, different subtypes of neurons are produced (represented in colors). A Notch binary fate decision further increases diversity of each progeny by producing two distinct fates (striped vs non-striped neurons). (c) Cross section of the developing larval optic lobe showing the sequential formation of the medulla. The first neuroblast produces the most posterior medulla column. Over time more neuroepithelial cells are converted into neuroblasts that will add columns to the medulla anteriorly. (d) Overlay of the temporal patterning of neuroblasts and the sequential production of medulla columns. (e and f) Larval (e) and adult (f) schematics of the medulla representing how the regionalization of the OPC increases neuronal diversity. The uni-columnar neuron Mi1 is produced all along the OPC independently of the spatial compartments, whereas the three multi-columnar neurons Pm1,2 and 3 are only produced by progenitors from specific domains.
Figure 4:
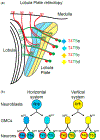
Retinotopic formation in the lobula plate: (a) Schematic of the lobula plate and the 4 subtypes of T4 (in red) and T5 neurons (in green). Each subtype targets to a different layer of the lobula plate neuropile (color coded for their response to motion direction, arrow on the side (b) Two neuroblasts give rise to the 4 T4s and T5s of a single column. Both go through two rounds of Notch mediated asymmetric decision and give rise to the 2 T4s and T5s of either the horizontal system for the Dpp+ neuroblast or of the vertical system for the Brk+ neuroblast.
Similar articles
- Spatio-temporal pattern of neuronal differentiation in the Drosophila visual system: A user's guide to the dynamic morphology of the developing optic lobe.
Ngo KT, Andrade I, Hartenstein V. Ngo KT, et al. Dev Biol. 2017 Aug 1;428(1):1-24. doi: 10.1016/j.ydbio.2017.05.008. Epub 2017 May 19. Dev Biol. 2017. PMID: 28533086 Free PMC article. - Temporal patterning of neurogenesis and neural wiring in the fly visual system.
Sato M, Yasugi T, Trush O. Sato M, et al. Neurosci Res. 2019 Jan;138:49-58. doi: 10.1016/j.neures.2018.09.009. Epub 2018 Sep 15. Neurosci Res. 2019. PMID: 30227165 Review. - Neuropil pattern formation and regulation of cell adhesion molecules in Drosophila optic lobe development depend on synaptobrevin.
Hiesinger PR, Reiter C, Schau H, Fischbach KF. Hiesinger PR, et al. J Neurosci. 1999 Sep 1;19(17):7548-56. doi: 10.1523/JNEUROSCI.19-17-07548.1999. J Neurosci. 1999. PMID: 10460261 Free PMC article. - Ebony protein in the Drosophila nervous system: optic neuropile expression in glial cells.
Richardt A, Rybak J, Störtkuhl KF, Meinertzhagen IA, Hovemann BT. Richardt A, et al. J Comp Neurol. 2002 Oct 7;452(1):93-102. doi: 10.1002/cne.10360. J Comp Neurol. 2002. PMID: 12205712 - From the Eye to the Brain: Development of the Drosophila Visual System.
Nériec N, Desplan C. Nériec N, et al. Curr Top Dev Biol. 2016;116:247-71. doi: 10.1016/bs.ctdb.2015.11.032. Epub 2016 Jan 20. Curr Top Dev Biol. 2016. PMID: 26970623 Free PMC article. Review.
Cited by
- Slit/Robo Signaling Regulates Multiple Stages of the Development of the Drosophila Motion Detection System.
Guzmán-Palma P, Contreras EG, Mora N, Smith M, González-Ramírez MC, Campusano JM, Sierralta J, Hassan BA, Oliva C. Guzmán-Palma P, et al. Front Cell Dev Biol. 2021 Apr 21;9:612645. doi: 10.3389/fcell.2021.612645. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33968921 Free PMC article. - Autophagy-dependent filopodial kinetics restrict synaptic partner choice during Drosophila brain wiring.
Kiral FR, Linneweber GA, Mathejczyk T, Georgiev SV, Wernet MF, Hassan BA, von Kleist M, Hiesinger PR. Kiral FR, et al. Nat Commun. 2020 Mar 12;11(1):1325. doi: 10.1038/s41467-020-14781-4. Nat Commun. 2020. PMID: 32165611 Free PMC article. - Tep1 Regulates Yki Activity in Neural Stem Cells in Drosophila Glioma Model.
Gangwani K, Snigdha K, Kango-Singh M. Gangwani K, et al. Front Cell Dev Biol. 2020 May 8;8:306. doi: 10.3389/fcell.2020.00306. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32457905 Free PMC article. - A Closer Look at Histamine in Drosophila.
Volonté C, Liguori F, Amadio S. Volonté C, et al. Int J Mol Sci. 2024 Apr 18;25(8):4449. doi: 10.3390/ijms25084449. Int J Mol Sci. 2024. PMID: 38674034 Free PMC article. Review. - Autocrine/Paracrine Slit-Robo Signaling Controls Optic Lobe Development in Drosophila melanogaster.
González-Ramírez MC, Rojo-Cortés F, Candia N, Garay-Montecinos J, Guzmán-Palma P, Campusano JM, Oliva C. González-Ramírez MC, et al. Front Cell Dev Biol. 2022 Jul 25;10:874362. doi: 10.3389/fcell.2022.874362. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35982851 Free PMC article.
References
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C: Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell 2003, 115:267–279. - PubMed
- Johnston RJ, Desplan C: Interchromosomal communication coordinates intrinsically stochastic expression between alleles. Science (80-) 2014, 343:661–665. - PMC - PubMed
- ••The establishment of the retinal mosaic is controled by the stochastic expression of the transcription factor Spineless (Ss). The authors show how the stochastic expression of ss is established at the level of individual ss alleles and how interchromosomal communication is required for coordinating the expression of both ss alleles.
- Schnaitmann C, Haikala V, Abraham E, Oberhauser V, Thestrup T, Griesbeck O, Reiff DF: Color Processing in the Early Visual System of Drosophila. Cell 2018, 172:318–318.e18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases