Peanut Sprout Extracts Attenuate Triglyceride Accumulation by Promoting Mitochondrial Fatty Acid Oxidation in Adipocytes - PubMed (original) (raw)
Peanut Sprout Extracts Attenuate Triglyceride Accumulation by Promoting Mitochondrial Fatty Acid Oxidation in Adipocytes
Seok Hee Seo et al. Int J Mol Sci. 2019.
Abstract
Peanut sprouts (PS), which are germinated peanut seeds, have recently been reported to have anti-oxidant, anti-inflammatory, and anti-obesity effects. However, the underlying mechanisms by which PS modulates lipid metabolism are largely unknown. To address this question, serial doses of PS extract (PSE) were added to 3T3-L1 cells during adipocyte differentiation. PSE (25 µg/mL) significantly attenuated adipogenesis by inhibiting lipid accumulation in addition to reducing the level of adipogenic protein and gene expression with the activation of AMP-activated protein kinase (AMPK). Other adipocyte cell models such as mouse embryonic fibroblasts C3H10T1/2 and primary adipocytes also confirmed the anti-adipogenic properties of PSE. Next, we investigated whether PSE attenuated lipid accumulation in mature adipocytes. We found that PSE significantly suppressed lipogenic gene expression, while fatty acid (FA) oxidation genes were upregulated. Augmentation of FA oxidation by PSE in mature 3T3-L1 adipocytes was confirmed via a radiolabeled-FA oxidation rate experiment by measuring the conversion of [³H]-oleic acid (OA) to [³H]-H₂O. Furthermore, PSE enhanced the mitochondrial oxygen consumption rate (OCR), especially maximal respiration, and beige adipocyte formation in adipocytes. In summary, PSE was effective in reducing lipid accumulation in 3T3-L1 adipocytes through mitochondrial fatty acid oxidation involved in AMPK and mitochondrial activation.
Keywords: adipogenesis; fatty acid oxidation; mitochondrial respiration; peanut sprouts; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
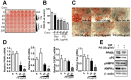
Effects of PSE on cell viability in 3T3-L1 and C3H10T1/2 pre-adipocytes. The culture of 3T3-L1 (A) and C3H1051/2 cells (B) were treated with 10‒100 μg/mL of PSE for 24 h. XTT reagent was added 3 h before measurement of OD 450 nm. Data are expressed as a percentage of the vehicle control (dimethyl sulfoxide (DMSO)). n.s. represents no significance. Data are represented as the mean ± SEM of three independent experiments. Values that do not share the same superscript are significantly different, as determined by one-way ANOVA (p < 0.05).
Figure 2
PSE inhibits adipogenesis in 3T3-L1 adipocytes. 3T3-L1 cells were seeded and induced to differentiation in the presence of DMSO (vehicle control), resveratrol (20–40 μM) or PSE (50–200 μg/mL) for 10 days: (A) TG accumulation in 96-well culture plates was visualized by ORO staining; (B) extracted ORO staining was quantified (OD 500 nm); (C) representative images from three separate experiments (magnified 4×); (D) adipogenic gene expression of PPARγ, aP2, C/EBP α, and Fas by qPCR; (E) adipogenic protein expressions of PPARγ, aP2, phosphor-specific, or total antibodies targeting AMPK and β-actin by Western blot analysis. All values are presented as the mean ±S.E.M. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 compared with the vehicle control (DMSO treated cells) by one-way ANOVA with Bonferroni’s comparison test. +; treatment, -; non-treatment.
Figure 3
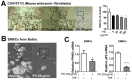
PSE inhibits adipogenesis in C3H10T1/2 mouse embryonic fibroblasts, adipocytes, and EMSCs. C3H10T1/2 cells were seeded and induced to differentiation in the presence of either DMSO (vehicle control) and PSE (5–100 μg/mL) for four days: (A) Triglyceride accumulation was visualized by Oil red-O staining and representative images from three separate experiments are shown in (left) (magnified 4×); extracted ORO staining was quantified (OD 500 nm) (right). Primary adipocytes were prepared from EMSC of Balb/c mice: (B) Phase contrast images of primary adipocytes were differentiated with or without PSE (25 μg/mL) for seven days (magnified 4×, scale bar = 400 μm); (C) adipogenic gene expression of PPARγ and aP2 by qPCR. * p < 0.05; ** p < 0.01; **** p < 0.0001 compared with the vehicle control (DMSO treated cells) by one-way ANOVA with Bonferroni’s comparison test or Student’s t-test. +; treatment, -; non-treatment.
Figure 4
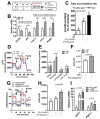
PSE attenuates lipid accumulation in cultures of adipocytes by upregulating fatty acid oxidation and mitochondrial oxygen consumption. (A) Experimental scheme. 3T3-L1 were seeded on the second day before differentiation (d-2) and induced to differentiation (d0, MDI: methyl isobutyl- xanthine, dexamethasone, and insulin). Keep 3T3-L1 cells differentiated into fully differentiated adipocytes until d7. Fully differentiated adipocytes (d7) were incubated with PSE (25 μg/mL) for three days. (B) Lipogenic and fatty acid oxidation-related gene expression of PPARγ, aP2, PGC1α, CPT1, and PPARα as determined by qPCR. (C) Conversion of [3H]-OA into [3H]-H2O. (D–F) Oxygen consumption rate (OCR) in 3T3-L1 adipocytes treated with Veh (blue) and PSE (red) as determined by Seahorse extracellular analyzer. 3T3-L1 cells differentiated into fully differentiated adipocytes. Fully differentiated adipocytes (d7) were incubated with PSE (25 μg/mL) for one day. Arrow indicates the addition of respiratory inhibitors of oligomycin (Oligo), carbonyl cyanide 4-trifluoromethoxy phenylhydrazone (FCCP) and a combination of antimycin A and rotenone (Rot/AA). (G–H) OCR in HepG2 cells treated with BSA (black), PA (blue), and PA + PSE (red) as determined by Seahorse extracellular analyzer. HepG2 cells were pre-incubated with PSE (50 μg/mL)for 48 h. BSA or 0.8 mM BSA-PA complex was loaded for 3 h. (I) Relative expressions of UCP1 and PGC1α by qPCR. Pre-treatment of the 3T3-L1 cell with PSE for 7 d during adipogenesis, followed by Bt2-cAMP stimulation for 6 h. All values are presented as the mean ±SEM. n.s. represents no significance. * p < 0.05; ** p < 0.01; *** p < 0.001 compared with the vehicle control (DMSO-treated cells) by Student’s _t_-test or one-way ANOVA with Bonferroni’s comparison test. Means that do not share a common superscript are significantly different as determined by one-way ANOVA with Bonferroni’s comparison test. ++ p < 0.01; +++ p < 0.001 compared with the vehicle control (DMSO treated cells) # p < 0.05; ## p < 0.01; ### p < 0.001 compared with PA-treated HepG2 cells by two-way ANOVA with Bonferroni’s comparison test. +; treatment, -; non-treatment.
Similar articles
- Peanut sprout rich in _p_-coumaric acid ameliorates obesity and lipopolysaccharide-induced inflammation and the inhibition of browning in adipocytes via mitochondrial activation.
Seo SH, Jo SM, Truong TTM, Zhang G, Kim DS, Lee M, Lee Y, Kang I. Seo SH, et al. Food Funct. 2021 Jun 21;12(12):5361-5374. doi: 10.1039/d1fo00342a. Food Funct. 2021. PMID: 33982705 - Gomisin N from Schisandra chinensis Ameliorates Lipid Accumulation and Induces a Brown Fat-Like Phenotype through AMP-Activated Protein Kinase in 3T3-L1 Adipocytes.
Lee K, Lee YJ, Kim KJ, Chei S, Jin H, Oh HJ, Lee BY. Lee K, et al. Int J Mol Sci. 2020 Mar 20;21(6):2153. doi: 10.3390/ijms21062153. Int J Mol Sci. 2020. PMID: 32245100 Free PMC article. - Kaempferol Isolated from Nelumbo nucifera Inhibits Lipid Accumulation and Increases Fatty Acid Oxidation Signaling in Adipocytes.
Lee B, Kwon M, Choi JS, Jeong HO, Chung HY, Kim HR. Lee B, et al. J Med Food. 2015 Dec;18(12):1363-70. doi: 10.1089/jmf.2015.3457. Epub 2015 Aug 17. J Med Food. 2015. PMID: 26280739 - Peroxisomal regulation of redox homeostasis and adipocyte metabolism.
Liu J, Lu W, Shi B, Klein S, Su X. Liu J, et al. Redox Biol. 2019 Jun;24:101167. doi: 10.1016/j.redox.2019.101167. Epub 2019 Mar 14. Redox Biol. 2019. PMID: 30921635 Free PMC article. Review. - The Lipid Side of Bone Marrow Adipocytes: How Tumor Cells Adapt and Survive in Bone.
Diedrich JD, Herroon MK, Rajagurubandara E, Podgorski I. Diedrich JD, et al. Curr Osteoporos Rep. 2018 Aug;16(4):443-457. doi: 10.1007/s11914-018-0453-9. Curr Osteoporos Rep. 2018. PMID: 29869753 Free PMC article. Review.
Cited by
- Peanut (Arachis hypogaea) sprout prevents high-fat diet-induced cognitive impairment by improving mitochondrial function.
Park SK, Lee HL, Kang JY, Kim JM, Heo HJ. Park SK, et al. Sci Rep. 2022 Apr 13;12(1):6213. doi: 10.1038/s41598-022-10520-5. Sci Rep. 2022. PMID: 35418581 Free PMC article. - Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice.
Seo SH, Fang F, Kang I. Seo SH, et al. Int J Environ Res Public Health. 2021 Jan 13;18(2):631. doi: 10.3390/ijerph18020631. Int J Environ Res Public Health. 2021. PMID: 33451038 Free PMC article. - Ginsenoside Rg3, enriched in red ginseng extract, improves lipopolysaccharides-induced suppression of brown and beige adipose thermogenesis with mitochondrial activation.
Feng F, Ko HA, Truong TMT, Song WJ, Ko EJ, Kang I. Feng F, et al. Sci Rep. 2024 Apr 22;14(1):9157. doi: 10.1038/s41598-024-59758-1. Sci Rep. 2024. PMID: 38644456 Free PMC article. - Inhalation of Patchouli (Pogostemon Cablin Benth.) Essential Oil Improved Metabolic Parameters in Obesity-Induced Sprague Dawley Rats.
Hong SJ, Cho J, Boo CG, Youn MY, Pan JH, Kim JK, Shin EC. Hong SJ, et al. Nutrients. 2020 Jul 13;12(7):2077. doi: 10.3390/nu12072077. Nutrients. 2020. PMID: 32668680 Free PMC article. - Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes.
Park HJ, Jo SM, Seo SH, Lee M, Lee Y, Kang I. Park HJ, et al. Int J Environ Res Public Health. 2020 Jun 30;17(13):4716. doi: 10.3390/ijerph17134716. Int J Environ Res Public Health. 2020. PMID: 32630030 Free PMC article.
References
- World Health Organization . Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. World Health Organization; Geneva, Switzerland: 2000. p. 253. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources