Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials - PubMed (original) (raw)
Meta-Analysis
Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials
Sidney M Rubinstein et al. BMJ. 2019.
Abstract
Objective: To assess the benefits and harms of spinal manipulative therapy (SMT) for the treatment of chronic low back pain.
Design: Systematic review and meta-analysis of randomised controlled trials.
Data sources: Medline, PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, Physiotherapy Evidence Database (PEDro), Index to Chiropractic Literature, and trial registries up to 4 May 2018, including reference lists of eligible trials and related reviews.
Eligibility criteria for selecting studies: Randomised controlled trials examining the effect of spinal manipulation or mobilisation in adults (≥18 years) with chronic low back pain with or without referred pain. Studies that exclusively examined sciatica were excluded, as was grey literature. No restrictions were applied to language or setting.
Review methods: Two reviewers independently selected studies, extracted data, and assessed risk of bias and quality of the evidence. The effect of SMT was compared with recommended therapies, non-recommended therapies, sham (placebo) SMT, and SMT as an adjuvant therapy. Main outcomes were pain and back specific functional status, examined as mean differences and standardised mean differences (SMD), respectively. Outcomes were examined at 1, 6, and 12 months. Quality of evidence was assessed using GRADE. A random effects model was used and statistical heterogeneity explored.
Results: 47 randomised controlled trials including a total of 9211 participants were identified, who were on average middle aged (35-60 years). Most trials compared SMT with recommended therapies. Moderate quality evidence suggested that SMT has similar effects to other recommended therapies for short term pain relief (mean difference -3.17, 95% confidence interval -7.85 to 1.51) and a small, clinically better improvement in function (SMD -0.25, 95% confidence interval -0.41 to -0.09). High quality evidence suggested that compared with non-recommended therapies SMT results in small, not clinically better effects for short term pain relief (mean difference -7.48, -11.50 to -3.47) and small to moderate clinically better improvement in function (SMD -0.41, -0.67 to -0.15). In general, these results were similar for the intermediate and long term outcomes as were the effects of SMT as an adjuvant therapy. Evidence for sham SMT was low to very low quality; therefore these effects should be considered uncertain. Statistical heterogeneity could not be explained. About half of the studies examined adverse and serious adverse events, but in most of these it was unclear how and whether these events were registered systematically. Most of the observed adverse events were musculoskeletal related, transient in nature, and of mild to moderate severity. One study with a low risk of selection bias and powered to examine risk (n=183) found no increased risk of an adverse event (relative risk 1.24, 95% confidence interval 0.85 to 1.81) or duration of the event (1.13, 0.59 to 2.18) compared with sham SMT. In one study, the Data Safety Monitoring Board judged one serious adverse event to be possibly related to SMT.
Conclusion: SMT produces similar effects to recommended therapies for chronic low back pain, whereas SMT seems to be better than non-recommended interventions for improvement in function in the short term. Clinicians should inform their patients of the potential risks of adverse events associated with SMT.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf and declare: SMR received personal grants from the European Chiropractors’ Union (ECU), the European Centre for Chiropractic Research Excellence (ECCRE), the Belgian Chiropractic Association (BVC) and the Netherlands Chiropractic Association (NCA) for his position at the Vrije Universiteit Amsterdam. He also received funding for a research project on chiropractic care for the elderly from the European Centre for Chiropractic Research and Excellence (ECCRE). AdeZ received a grant from the European Chiropractors’ Union (ECU) (grant No A14.03), for an independent study on the effects of SMT. SMR and AdeZ declare that they work in their own private clinics as chiropractors. The remaining authors received no support from any organisation for the submitted work; have no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and have no other relationships or activities that could appear to have influenced the submitted work.
Figures
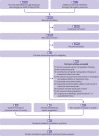
Fig 1
Selection of studies through review
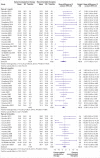
Fig 2
Mean difference in reduction of pain at 1, 3, 6, and 12 months (0-100; 0=no pain, 100 maximum pain) for spinal manipulative therapy (SMT) versus recommended therapies in review of the effects of SMT for chronic low back pain. Pooled mean differences calculated by DerSimonian-Laird random effects model. See supplementary file for more detailed graphic
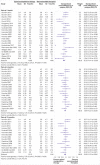
Fig 3
Standardised mean difference for improvement in function at 1, 3, 6, and 12 months for spinal manipulative therapy (SMT) versus recommended therapies in review of the effects of SMT for chronic low back pain. Pooled standardised mean differences calculated by DerSimonian-Laird random effects model. See supplementary file for more detailed graphic
Similar articles
- Spinal manipulative therapy for acute low-back pain.
Rubinstein SM, Terwee CB, Assendelft WJ, de Boer MR, van Tulder MW. Rubinstein SM, et al. Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD008880. doi: 10.1002/14651858.CD008880.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972127 Free PMC article. Review. - Spinal manipulative therapy for chronic low-back pain.
Rubinstein SM, van Middelkoop M, Assendelft WJ, de Boer MR, van Tulder MW. Rubinstein SM, et al. Cochrane Database Syst Rev. 2011 Feb 16;(2):CD008112. doi: 10.1002/14651858.CD008112.pub2. Cochrane Database Syst Rev. 2011. PMID: 21328304 Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Association of Spinal Manipulative Therapy With Clinical Benefit and Harm for Acute Low Back Pain: Systematic Review and Meta-analysis.
Paige NM, Miake-Lye IM, Booth MS, Beroes JM, Mardian AS, Dougherty P, Branson R, Tang B, Morton SC, Shekelle PG. Paige NM, et al. JAMA. 2017 Apr 11;317(14):1451-1460. doi: 10.1001/jama.2017.3086. JAMA. 2017. PMID: 28399251 Free PMC article. Review. - Spinal manipulative therapy for chronic low-back pain: an update of a Cochrane review.
Rubinstein SM, van Middelkoop M, Assendelft WJ, de Boer MR, van Tulder MW. Rubinstein SM, et al. Spine (Phila Pa 1976). 2011 Jun;36(13):E825-46. doi: 10.1097/BRS.0b013e3182197fe1. Spine (Phila Pa 1976). 2011. PMID: 21593658 Review.
Cited by
- Presence of Tumor Necrosis Factor-Alpha in Urine Samples of Patients With Chronic Low Back Pain Undergoing Chiropractic Care: Preliminary Findings From a Prospective Cohort Study.
Gevers-Montoro C, Romero-Santiago M, Losapio L, Conesa-Buendía FM, Newell D, Álvarez-Galovich L, Piché M, Ortega-De Mues A. Gevers-Montoro C, et al. Front Integr Neurosci. 2022 Apr 12;16:879083. doi: 10.3389/fnint.2022.879083. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35492573 Free PMC article. - Spinal manipulative therapy in older adults with chronic low back pain: an individual participant data meta-analysis.
Jenks A, de Zoete A, van Tulder M, Rubinstein SM; International IPD-SMT group. Jenks A, et al. Eur Spine J. 2022 Jul;31(7):1821-1845. doi: 10.1007/s00586-022-07210-1. Epub 2022 May 28. Eur Spine J. 2022. PMID: 35633383 Review. - High-definition transcranial infraslow pink noise stimulation for chronic low back pain: protocol for a pilot, safety and feasibility randomised placebo-controlled trial.
Adhia DB, Mani R, Reynolds JNJ, Vanneste S, De Ridder D. Adhia DB, et al. BMJ Open. 2022 Jun 15;12(6):e056842. doi: 10.1136/bmjopen-2021-056842. BMJ Open. 2022. PMID: 35705354 Free PMC article. - Investigating the hypoalgesic effects of spinal manipulative therapy using hidden pain conditioning and positive expectation in patients with chronic low back pain: protocol for a randomised controlled trial.
Nogueira Carrer HC, Lima TC, George SZ, Reis FJJD, Dias DLC, Campanha BES, Chaves TC. Nogueira Carrer HC, et al. BMJ Open. 2023 Apr 12;13(4):e066199. doi: 10.1136/bmjopen-2022-066199. BMJ Open. 2023. PMID: 37045570 Free PMC article. - Beyond exercise. Can application of manual therapy before exercise benefit a low functioning person with limb loss? A case study.
Wong CK, Youdan GA, Chihuri ST. Wong CK, et al. J Man Manip Ther. 2023 Oct;31(5):383-389. doi: 10.1080/10669817.2023.2192650. Epub 2023 Mar 21. J Man Manip Ther. 2023. PMID: 36942674 Free PMC article.
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
- Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 2017;166:514-30. 10.7326/M16-2367 - DOI - PubMed
- Bons SCS, Borg MAJP, Van den Donk M, et al. NHG guideline for aspecific low-back pain, 2017. www.nhgorg/standaarden/samenvatting/aspecifieke-lagerugpijn#idp23613872.
- NICE guideline. Low back pain and sciatica in over 16s: assessment and management. www.nice.org.uk/guidance/NG59/chapter/Recommendations#non-invasive-treat..., 2016. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical