LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway - PubMed (original) (raw)
LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway
Hongrong Li et al. Lipids Health Dis. 2019.
Abstract
Background: Endothelial-to-mesenchymal transition (EndMT) plays significant roles in atherosclerosis, but the regulatory mechanisms involving lncRNAs remain to be elucidated. Here we sort to identify the role of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in ox-LDL-induced EndMT.
Methods: The atherosclerosis model was established by feeding ApoE-/- mice with high-fat diet, and the levels of lncRNA MALAT1 in mouse arterial tissue were detected by RT-qPCR. Cell model was established by treating human umbilical vein endothelial cells (HUVECs) with ox-LDL, and the levels of EndMT markers, such as CD31, vWF, α-SMA and Vimentin and lncRNA MALAT1 levels were detected and their correlations were analyzed. The role of MALAT1 in EndMT and its dependence on Wnt/β-catenin signaling pathway was further detected by knocking down or overexpressing MALAT1.
Results: MALAT1 was upregulated in high-fat food fed ApoE-/- mice. HUVECs treated with ox-LDL showed a significant decrease in expression of CD31 and vWF, a significant increase in expression of α-SMA and vimentin, and upregulated MALAT1. An increased MALAT1 level facilitated the nuclear translocation of β-catenin induced by ox-LDL. Inhibition of MALAT1 expression reversed nuclear translocation of β-catenin and EndMT. Moreover, overexpression of MALAT1 enhanced the effects of ox-LDL on HUVEC EndMT and Wnt/β-catenin signaling activation.
Conclusions: Our study revealed that the pathological EndMT required the activation of the MALAT1-dependent Wnt/β-catenin signaling pathway, which may be important for the onset of atherosclerosis.
Trial registration: Not applicable.
Keywords: Atherosclerosis; EndMT; MALAT1; Ox-LDL; Wnt/β-catenin.
Conflict of interest statement
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All animal experiments were conducted according to the ethical guidelines of Animal Ethics Committee of Hebei Medical University.
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
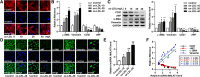
Changes of serum lipid, histology and MALAT1 in mice**.** _ApoE_−/− mice were fed with HFD for 16 weeks, and normally fed C57B/6 mice were used as control. a Serum lipid levels of the two groups were detected by automatic biochemical analyzer (n = 9, **P < 0.01, versus control group). b H&E staining of vessel wall. Magnification, 200x. c Level of MALAT1 in aortas tissues as determined by qRT-PCR (n = 3, **P < 0.01, versus control group)
Fig. 2
Changes of cellular morphology, EndMT markers and MALAT1 in HUVECs. HUVECs were treated with ox-LDL at different concentrations (10, 20, 40 μg/ml) for 48 h. a Cellular F-actin was stained with rhodamine phalloidin. Nuclei were stained with DAPI Fluoromount-G®. Cells were observed under fluorescence microscopy. Magnification, 400x. b-d Endothelial markers (CD31, vWF) and mesenchymal markers (α-SMA, vimentin) were detected by qRT-PCR (B), Western blot (C) (n = 3, *P < 0.05, **P < 0.01, versus control group) and immunofluorescence analysis (D). Magnification, 400x. e MALAT1 expression was detected by qRT-PCR (n = 3, *P < 0.05, **P < 0.01 versus control group). The relative levels of α-SMA, vimentin, CD31, vWF and MALAT1 in ox-LDL groups were normalized to those in control group, respectively. f Pearson correlations between MALAT1 and α-SMA mRNA, vimentin mRNA, CD31 mRNA, vWF mRNA
Fig. 3
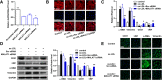
Knockdown of MALAT1 attenuates ox-LDL-induced EndMT in HUVECs. a HUVECs were transfected with MALAT1-siRNA1, MALAT1-siRNA2, MALAT1-siRNA3 or scramble control (scr) for 24 h. The MALAT1 levels were determined by qRT-PCR (n = 3, *P < 0.05, versus negative control group). b The effect of MALAT1-siRNA on cell morphology induced by ox-LDL (40 μg/ml) was observed through rhodamine phalloidin-stained F-actin. Magnification, 400x. c-e The effect of MALAT1-siRNA3 on the expression of endothelial markers (CD31, vWF) and mesenchymal markers (α-SMA, vimentin) were detected by qRT-PCR (c), Western blot (d) (n = 3, *P < 0.05, **P < 0.01 versus control group, #P < 0.05, ##P < 0.01 versus ox-LDL group) and immunofluorescence analysis (e). Magnification, 400x
Fig. 4
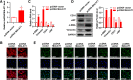
The effect of MALAT1 overexpression on EndMT of HUVECs. Full-length human MALAT1 cDNA plasmid (pcDNA-MALAT1) was constructed and transfected into HUVECs. The empty vector was used as negative control. a MALAT1 expression was detected by qRT-PCR (n = 3, *P < 0.05, versus pcDNA vector group). b The effect of MALAT1 overexpression on cell morphology induced by ox-LDL (40 μg/ml) was observed through rhodamine phalloidin-stained F-actin. Magnification, 400x. c-e The effect of MALAT1 overexpression on the expression of endothelial markers (CD31, vWF) and mesenchymal markers (α-SMA, vimentin) were detected by qRT-PCR (c), Western blot (d) (n = 3, *P < 0.05, **P < 0.01 versus pcDNA vector group) and immunofluorescence analysis (e). Magnification, 400x
Fig. 5
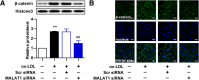
Knockdown of MALAT1 inhibits ox-LDL-induced β-catenin translocation. HUVECs were transfected with MALAT1-siRNA or scramble control (scr) for 24 h. a The protein expression of β-catenin in nucleus (n = 3, *P < 0.05, versus control group, #P < 0.05, versus model group) was analyze by Western blot. b The location of β-catenin was detected by immunofluorescence analysis. Magnification, 400x
Fig. 6
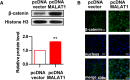
Overexpression of MALAT1 promotes activation of Wnt/β-catenin pathway. HUVECs were transfected with pcDNA-MALAT1 or pcDNA vector for 48 h. a The protein level of β-catenin in nucleus (n = 3, *P < 0.05, versus pcDNA vector group) was analyze by Western blot. b The location of β-catenin was detected by immunofluorescence analysis. Magnification, 400x
Similar articles
- lncRNA ZFAS1 promotes ox-LDL induced EndMT through miR-150-5p/Notch3 signaling axis.
Yin Q, He M, Huang L, Zhang X, Zhan J, Hu J. Yin Q, et al. Microvasc Res. 2021 Mar;134:104118. doi: 10.1016/j.mvr.2020.104118. Epub 2020 Dec 2. Microvasc Res. 2021. PMID: 33278458 - Knockdown of LINC00657 inhibits ox-LDL-induced endothelial cell injury by regulating miR-30c-5p/Wnt7b/β-catenin.
Wu H, Liu T, Hou H. Wu H, et al. Mol Cell Biochem. 2020 Sep;472(1-2):145-155. doi: 10.1007/s11010-020-03793-9. Epub 2020 Jun 23. Mol Cell Biochem. 2020. PMID: 32577947 - H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/β-catenin in ox-LDL -stimulated vascular smooth muscle cells.
Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R. Zhang L, et al. J Biomed Sci. 2018 Feb 7;25(1):11. doi: 10.1186/s12929-018-0418-4. J Biomed Sci. 2018. PMID: 29415742 Free PMC article. - Endothelial to Mesenchymal Transition in the Cardiogenesis and Cardiovascular Diseases.
Anbara T, Sharifi M, Aboutaleb N. Anbara T, et al. Curr Cardiol Rev. 2020;16(4):306-314. doi: 10.2174/1573403X15666190808100336. Curr Cardiol Rev. 2020. PMID: 31393254 Free PMC article. Review. - Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases.
Piera-Velazquez S, Jimenez SA. Piera-Velazquez S, et al. Physiol Rev. 2019 Apr 1;99(2):1281-1324. doi: 10.1152/physrev.00021.2018. Physiol Rev. 2019. PMID: 30864875 Free PMC article. Review.
Cited by
- The Journey of Cancer Cells to the Brain: Challenges and Opportunities.
Łazarczyk M, Mickael ME, Skiba D, Kurzejamska E, Ławiński M, Horbańczuk JO, Radziszewski J, Fraczek K, Wolinska R, Paszkiewicz J, Religa P, Sacharczuk M. Łazarczyk M, et al. Int J Mol Sci. 2023 Feb 14;24(4):3854. doi: 10.3390/ijms24043854. Int J Mol Sci. 2023. PMID: 36835266 Free PMC article. Review. - The Role and Research Progress of Inhibitor of Differentiation 1 in Atherosclerosis.
Qiu J, Li Y, Wang B, Sun X, Qian D, Ying Y, Zhou J. Qiu J, et al. DNA Cell Biol. 2022 Feb;41(2):71-79. doi: 10.1089/dna.2021.0745. Epub 2022 Jan 19. DNA Cell Biol. 2022. PMID: 35049366 Free PMC article. Review. - The Combined Regulation of Long Non-coding RNA and RNA-Binding Proteins in Atherosclerosis.
Ding Y, Yin R, Zhang S, Xiao Q, Zhao H, Pan X, Zhu X. Ding Y, et al. Front Cardiovasc Med. 2021 Nov 2;8:731958. doi: 10.3389/fcvm.2021.731958. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34796209 Free PMC article. Review. - RAGE signaling regulates the progression of diabetic complications.
Taguchi K, Fukami K. Taguchi K, et al. Front Pharmacol. 2023 Mar 16;14:1128872. doi: 10.3389/fphar.2023.1128872. eCollection 2023. Front Pharmacol. 2023. PMID: 37007029 Free PMC article. Review. - Roles of LncRNAs in the Pathogenesis of Pulmonary Hypertension.
Liu T, Xu S, Yang J, Xing X. Liu T, et al. Rev Cardiovasc Med. 2024 Jun 17;25(6):217. doi: 10.31083/j.rcm2506217. eCollection 2024 Jun. Rev Cardiovasc Med. 2024. PMID: 39076325 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous